Summary
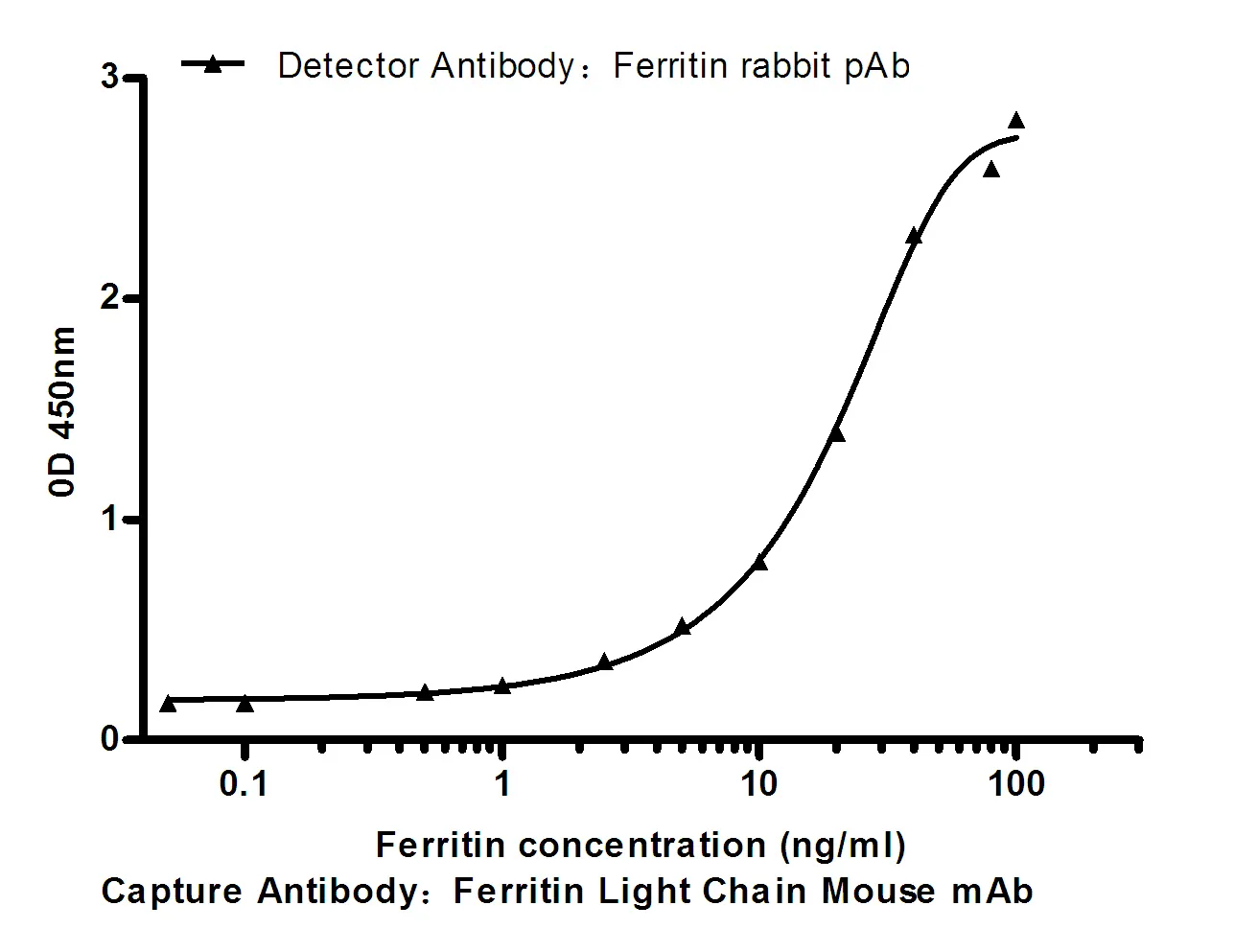
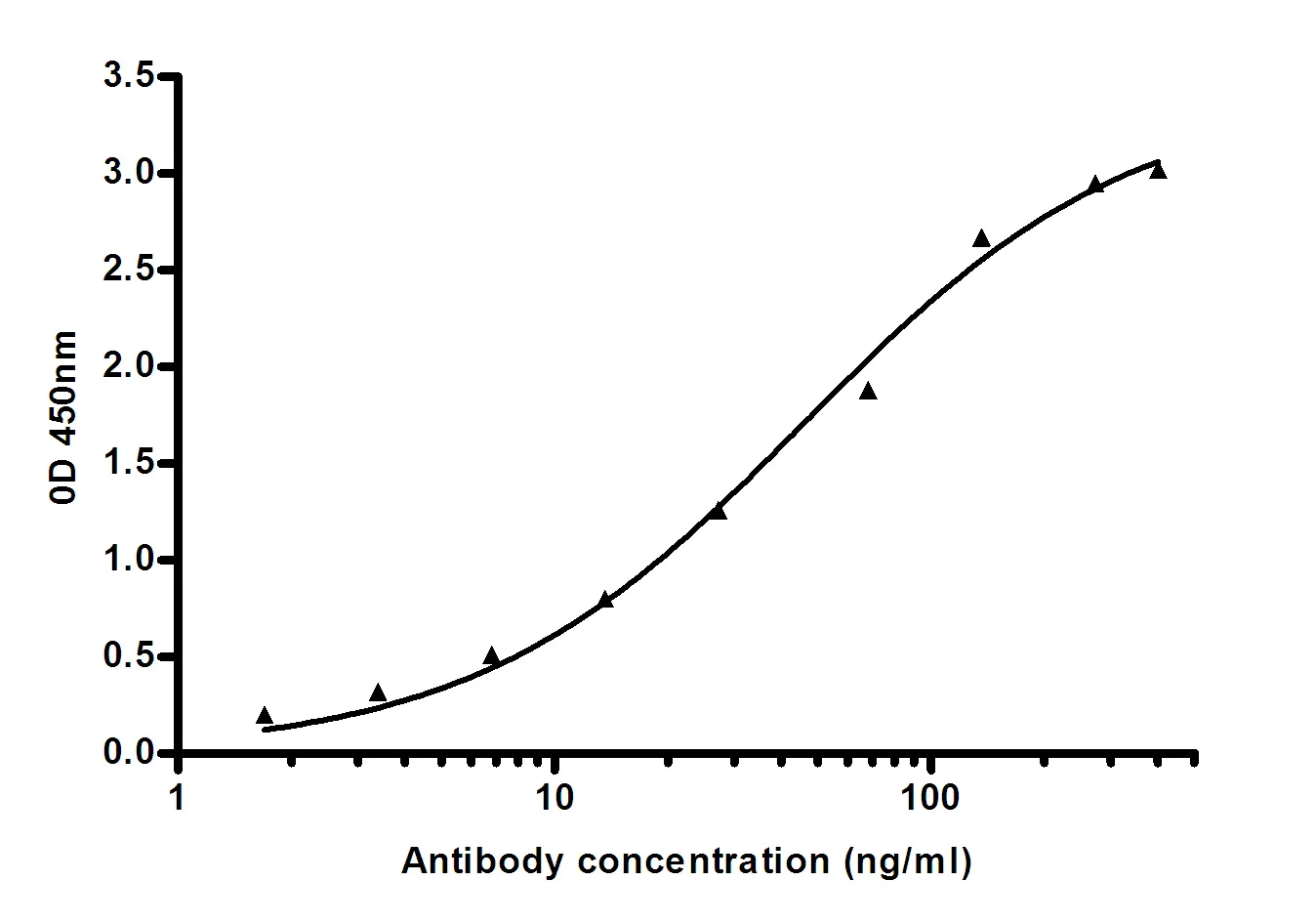
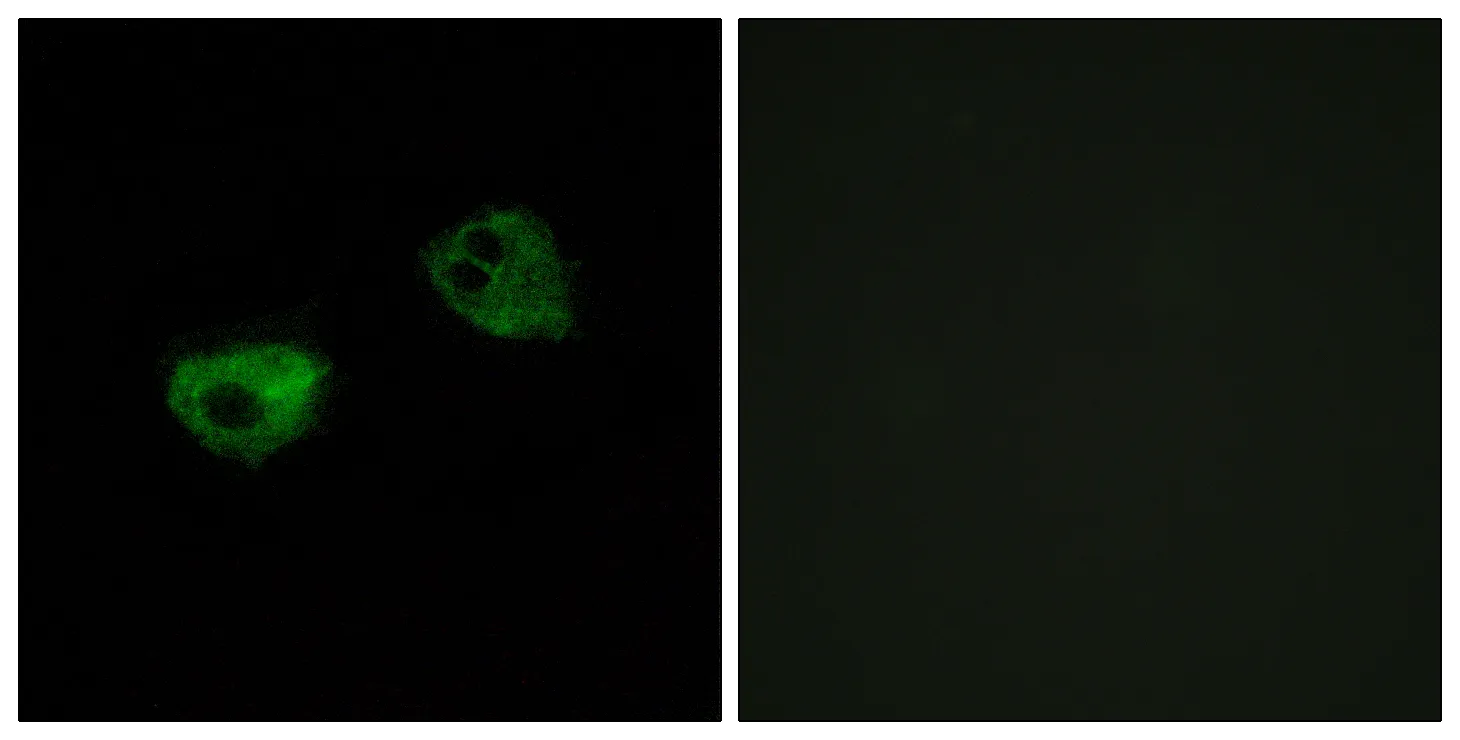
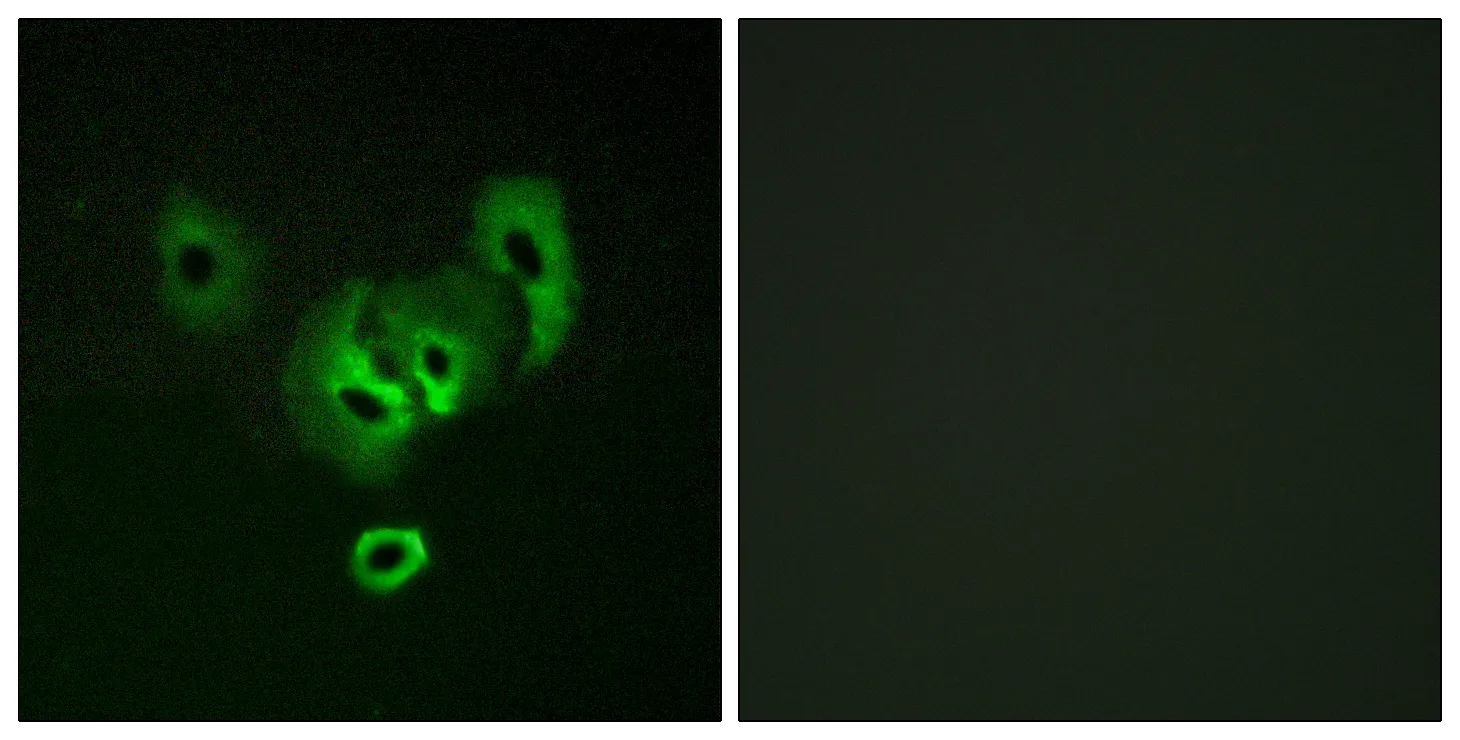
Performance
Immunogen
Application
Background
This gene encodes the subunit of a collagen-like molecule associated with acetylcholinesterase in skeletal muscle. Each molecule is composed of three identical subunits. Each subunit contains a proline-rich attachment domain (PRAD) that binds an acetylcholinesterase tetramer to anchor the catalytic subunit of the enzyme to the basal lamina. Mutations in this gene are associated with endplate acetylcholinesterase deficiency. Multiple transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Jul 2008],disease:Defects in COLQ are the cause of congenital myasthenic syndrome Engel type (CMSE) [MIM:603034]; also known as end-plate acetylcholinesterase deficiency or congenital myasthenic syndrome type IC (CMS-IC). CMSE is a rare autosomal recessive congenital myastehnic syndrome characterized by onset during childhood, generalized weakness, abnormal fatigability on exertion, refrectoriness to acetylcholinesterase drugs, decremental electromyographic response and morphological abnormalities of the neuromuscular junctions.,domain:The proline-rich attachment domain (PRAD) binds the AChE catalytic subunits.,function:Anchors the catalytic subunits of asymmetric AChE to the synaptic basal lamina.,PTM:The triple-helical tail is stabilized by disulfide bonds at each end.,similarity:Belongs to the COLQ family.,similarity:Contains 2 collagen-like domains.,subunit:Homotrimer. Component of the asymmetric form of AChE, a disulfide-bonded oligomer composed of the collagenic subunits (Q) and a variable number of asymmetric catalytic subunits (T). The N-terminal of a collagenic subunit (Q) associates with the C-terminal of a catalytic subunit (T).,tissue specificity:Found at the end plate of skeletal muscle.,
Research Area