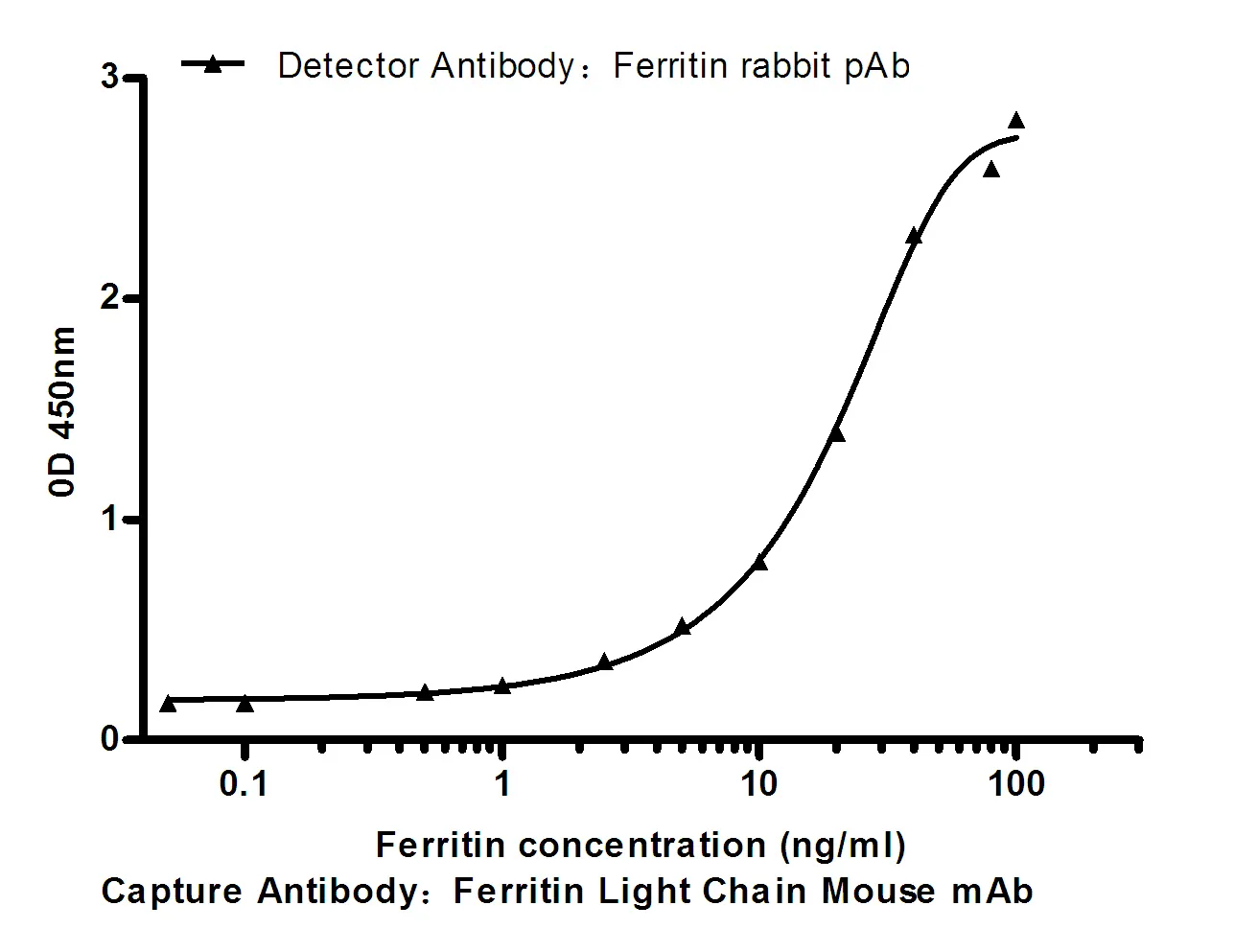
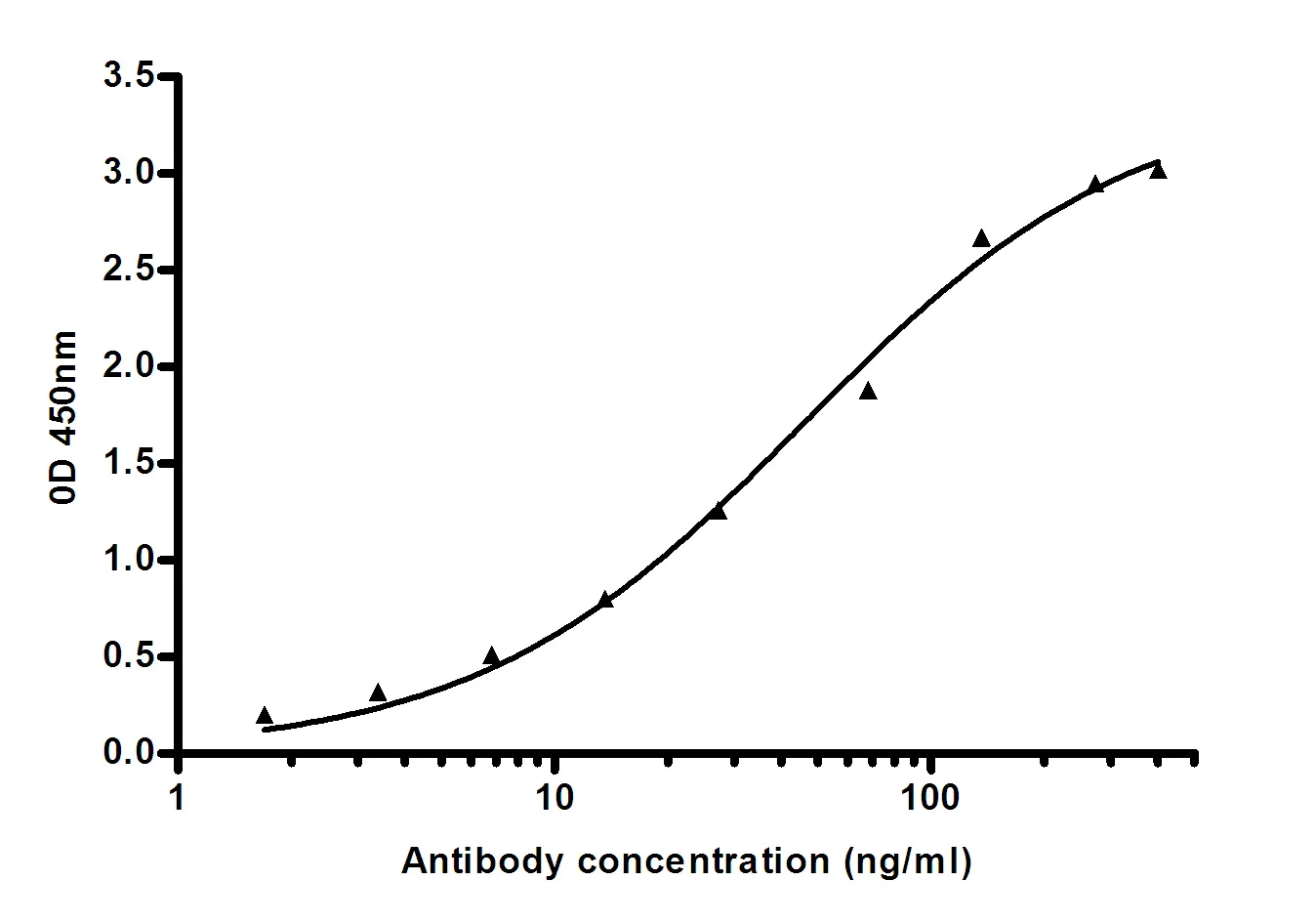
Summary
Performance
Immunogen
Application
Background
The human NDUFA1 gene codes for an essential component of complex I of the respiratory chain, which transfers electrons from NADH to ubiquinone. It has been noted that the N-terminal hydrophobic domain has the potential to be folded into an alpha-helix spanning the inner mitochondrial membrane with a C-terminal hydrophilic domain interacting with globular subunits of complex I. The highly conserved two-domain structure suggests that this feature is critical for the protein function and might act as an anchor for the NADH:ubiquinone oxidoreductase complex at the inner mitochondrial membrane. However, the NDUFA1 peptide is one of about 31 components of the "hydrophobic protein" (HP) fraction of complex I which is involved in proton translocation. Thus the NDUFA1 peptide may also participate in that function. [provided by RefSeq, Jul 2008],disease:Defects in NDUFA1 are a cause of complex I mitochondrial respiratory chain deficiency [MIM:252010]. Complex I (NADH-ubiquinone oxidoreductase), the largest complex of the mitochondrial respiratory chain, contains more than 40 subunits. It is embedded in the inner mitochondrial membrane and is partly protruding in the matrix. Complex I deficiency is the most common cause of mitochondrial disorders. It represents largely one-third of all cases of respiratory chain deficiency and is responsible for a variety of clinical symptoms, ranging from neurological disorders to cardiomyopathy, liver failure, and myopathy.,function:Accessory subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase (Complex I), that is believed to be not involved in catalysis. Complex I functions in the transfer of electrons from NADH to the respiratory chain. The immediate electron acceptor for the enzyme is believed to be ubiquinone.,similarity:Belongs to the complex I NDUFA1 subunit family.,subunit:Complex I is composed of 45 different subunits.,tissue specificity:Primarily expressed in heart and skeletal muscle.,
Research Area