Summary
Performance
Immunogen
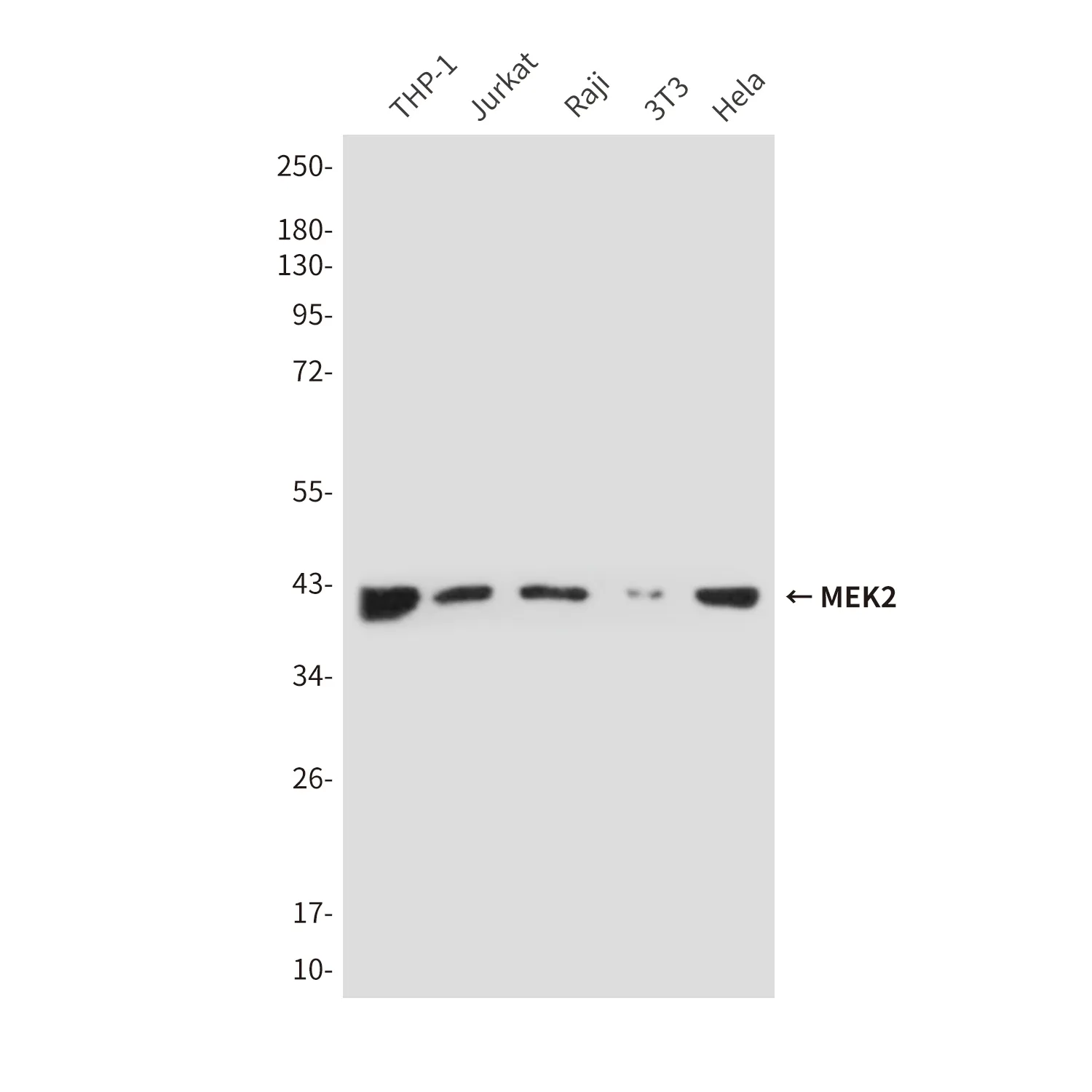
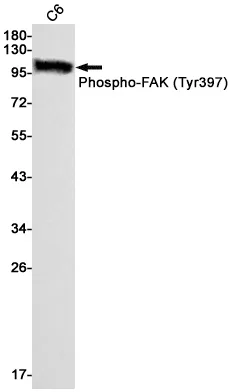
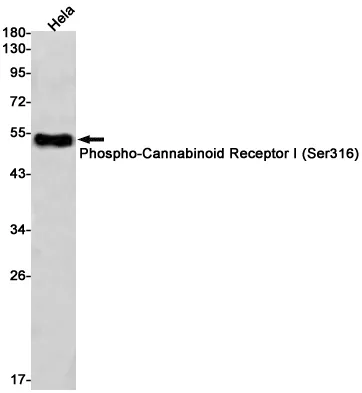
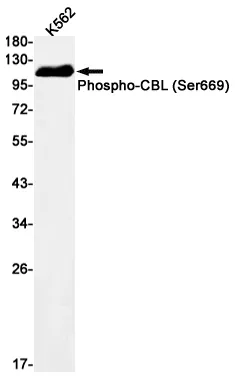
Application
Background
Catalyzes the formation of aromatic C18 estrogens from C19 androgens. A cytochrome P450 monooxygenase that catalyzes the conversion of C19 androgens, androst-4-ene-3,17-dione (androstenedione) and testosterone to the C18 estrogens, estrone and estradiol, respectively (PubMed:27702664, PubMed:2848247). Catalyzes three successive oxidations of C19 androgens: two conventional oxidations at C19 yielding 19-hydroxy and 19-oxo/19-aldehyde derivatives, followed by a third oxidative aromatization step that involves C1-beta hydrogen abstraction combined with cleavage of the C10-C19 bond to yield a phenolic A ring and formic acid (PubMed:20385561). Alternatively, the third oxidative reaction yields a 19-norsteroid and formic acid. Converts dihydrotestosterone to delta1,10-dehydro 19- nordihydrotestosterone and may play a role in homeostasis of this potent androgen (PubMed:22773874). Also displays 2-hydroxylase activity toward estrone (PubMed:22773874). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (CPR; NADPH-ferrihemoprotein reductase) (PubMed:20385561, PubMed:22773874).
Research Area