Summary
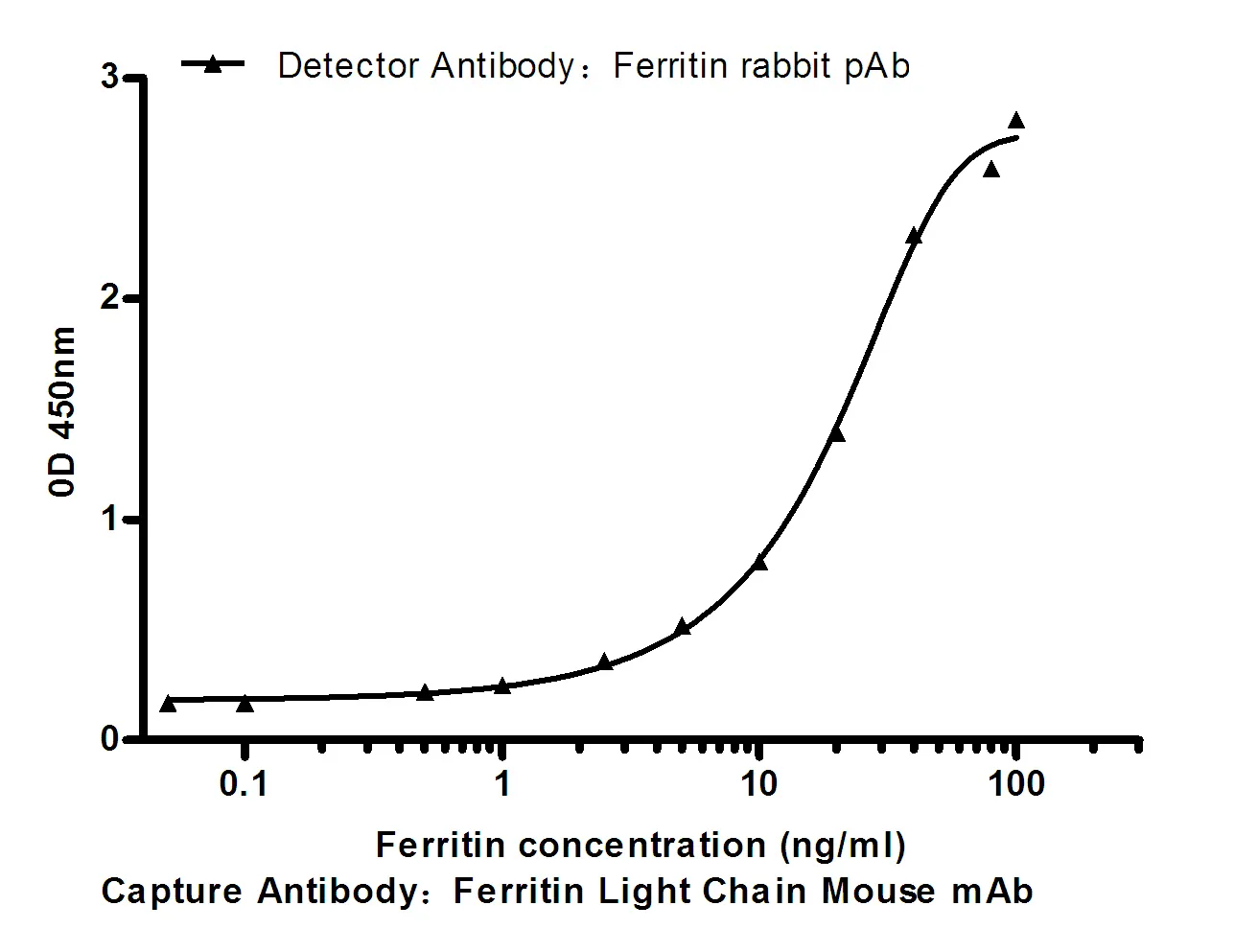
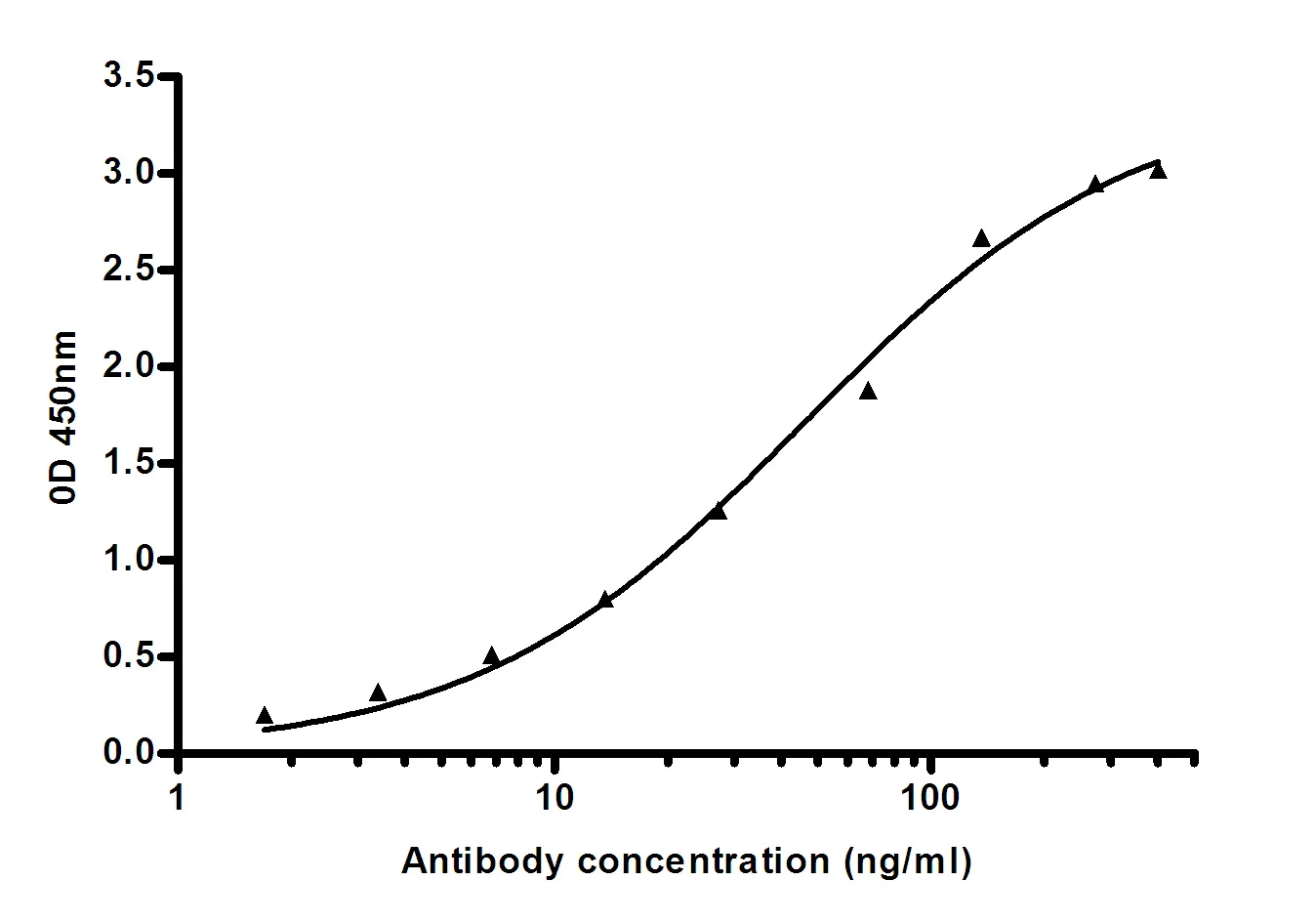
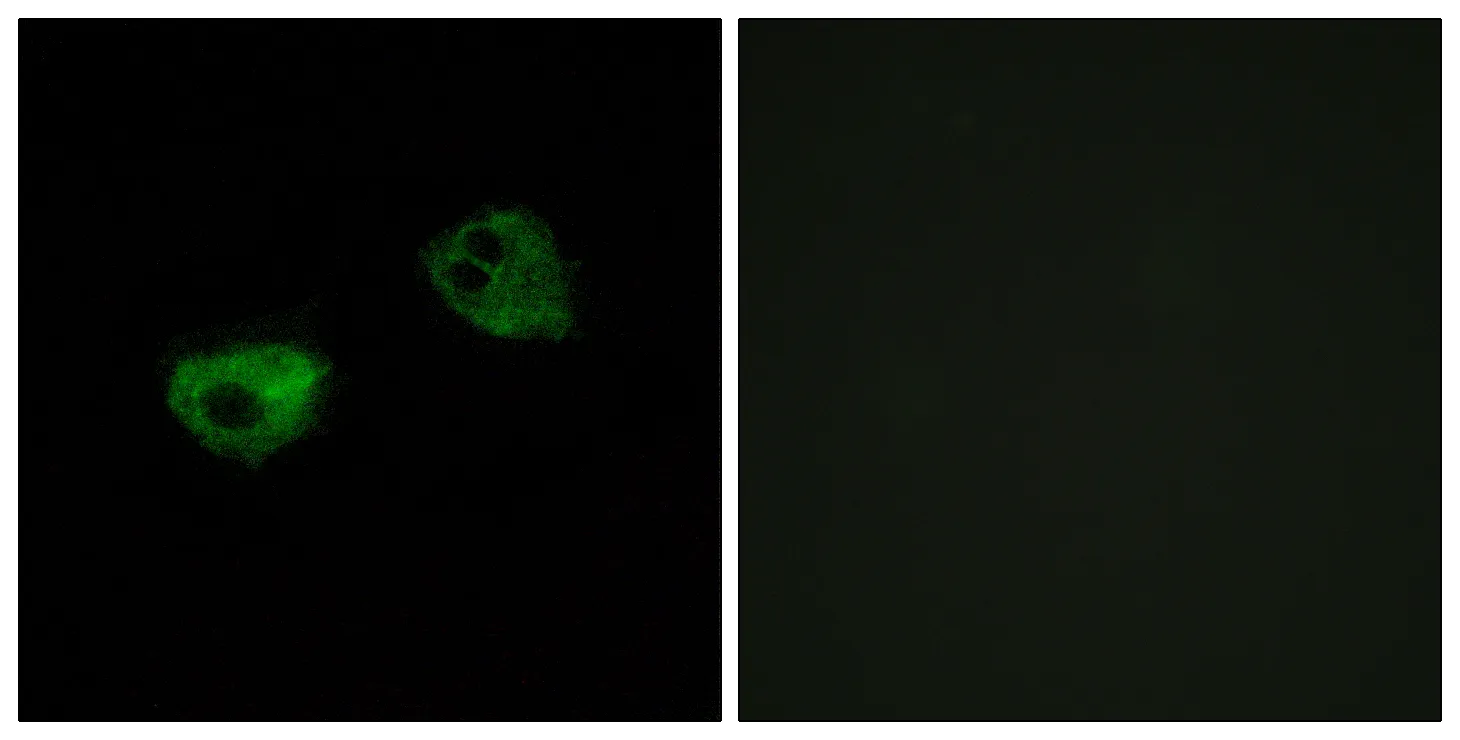
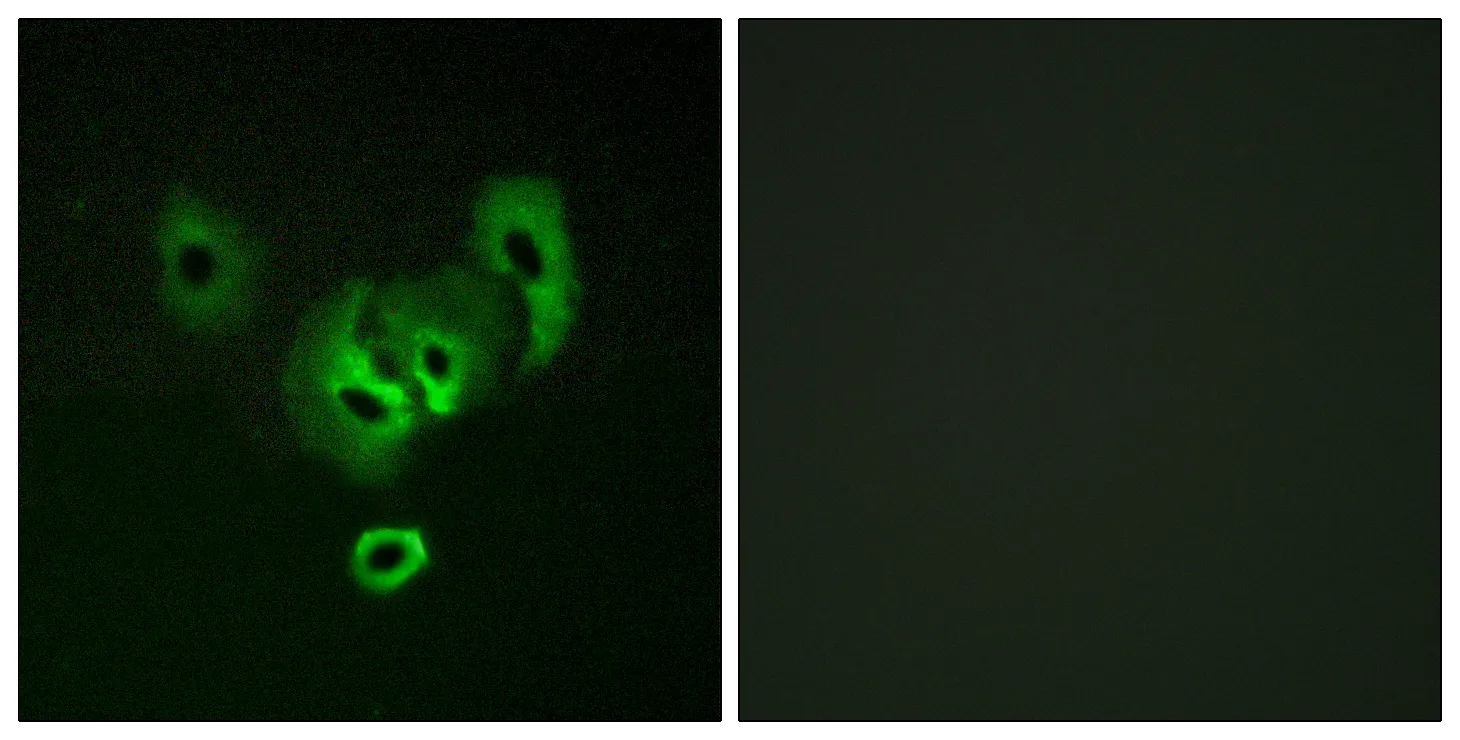
Performance
Immunogen
Application
Background
domain:The RING finger domain, in addition to its role in ubiquitination, functions as a structural scaffold to bring two clusters of positive-charged residues within spatial proximity to mimic a bipartite nuclear localization signal (NLS).,function:E3 ubiquitin-protein ligase that mediates ubiquitination and subsequent proteasomal degradation of target proteins. E3 ubiquitin ligases accept ubiquitin from an E2 ubiquitin-conjugating enzyme in the form of a thioester and then directly transfers the ubiquitin to targeted substrates. Involved in JUN ubiquitination and degradation. Directly involved in p53 (TP53) ubiquitination and degradation, thereby abolishing p53-dependent transcription and apoptosis. Ubiquitinates p53 independently of MDM2 or RCHY1. Probably mediates E3 ubiquitin ligase activity by functioning as the essential RING domain subunit of larger E3 complexes. In contrast, it does not constitute the catalytic RING subunit in the DCX DET1-COP1 complex that negatively regulates JUN, the ubiquitin ligase activity being mediated by RBX1.,induction:By p53/TP53.,pathway:Protein modification; protein ubiquitination.,similarity:Belongs to the COP1 family.,similarity:Contains 1 RING-type zinc finger.,similarity:Contains 7 WD repeats.,subcellular location:In the nucleus, it forms nuclear speckles.,subunit:Homodimer. Homodimerization is mediated by the coiled coil domain. Component of the DCX DET1-COP1 ubiquitin ligase complex at least composed of RBX1, DET1, DDB1, CUL4A and COP1. Isoform 2 does not interact with CUL4A but still binds to RBX1, suggesting that the interaction may be mediated by another culllin protein. Isoform 1 and isoform 2 interact with CUL5 but not with CUL1, CUL2 not CUL3. Interacts with bZIP transcription factors JUN, JUNB and JUND but not with FOS, ATF2 nor XBP1. Interacts with p53 (TP53).,tissue specificity:Ubiquitously expressed at low level. Expressed at higher level in testis, placenta, skeletal muscle and heart.,domain:The RING finger domain, in addition to its role in ubiquitination, functions as a structural scaffold to bring two clusters of positive-charged residues within spatial proximity to mimic a bipartite nuclear localization signal (NLS).,function:E3 ubiquitin-protein ligase that mediates ubiquitination and subsequent proteasomal degradation of target proteins. E3 ubiquitin ligases accept ubiquitin from an E2 ubiquitin-conjugating enzyme in the form of a thioester and then directly transfers the ubiquitin to targeted substrates. Involved in JUN ubiquitination and degradation. Directly involved in p53 (TP53) ubiquitination and degradation, thereby abolishing p53-dependent transcription and apoptosis. Ubiquitinates p53 independently of MDM2 or RCHY1. Probably mediates E3 ubiquitin ligase activity by functioning as the essential RING domain subunit of larger E3 complexes. In contrast, it does not constitute the catalytic RING subunit in the DCX DET1-COP1 complex that negatively regulates JUN, the ubiquitin ligase activity being mediated by RBX1.,induction:By p53/TP53.,pathway:Protein modification; protein ubiquitination.,similarity:Belongs to the COP1 family.,similarity:Contains 1 RING-type zinc finger.,similarity:Contains 7 WD repeats.,subcellular location:In the nucleus, it forms nuclear speckles.,subunit:Homodimer. Homodimerization is mediated by the coiled coil domain. Component of the DCX DET1-COP1 ubiquitin ligase complex at least composed of RBX1, DET1, DDB1, CUL4A and COP1. Isoform 2 does not interact with CUL4A but still binds to RBX1, suggesting that the interaction may be mediated by another culllin protein. Isoform 1 and isoform 2 interact with CUL5 but not with CUL1, CUL2 not CUL3. Interacts with bZIP transcription factors JUN, JUNB and JUND but not with FOS, ATF2 nor XBP1. Interacts with p53 (TP53).,tissue specificity:Ubiquitously expressed at low level. Expressed at higher level in testis, placenta, skeletal muscle and heart.,
Research Area
p53;Ubiquitin mediated proteolysis;