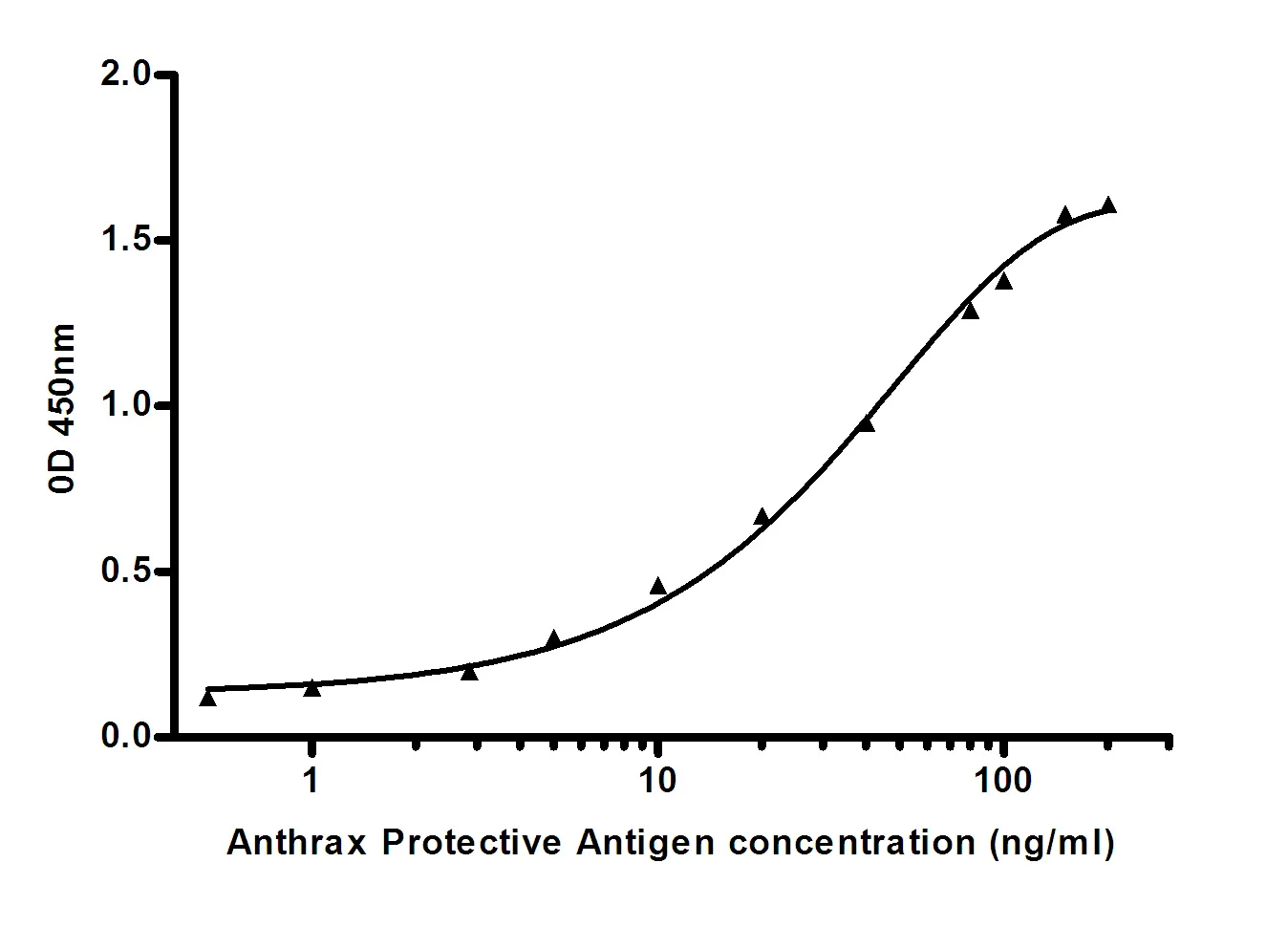
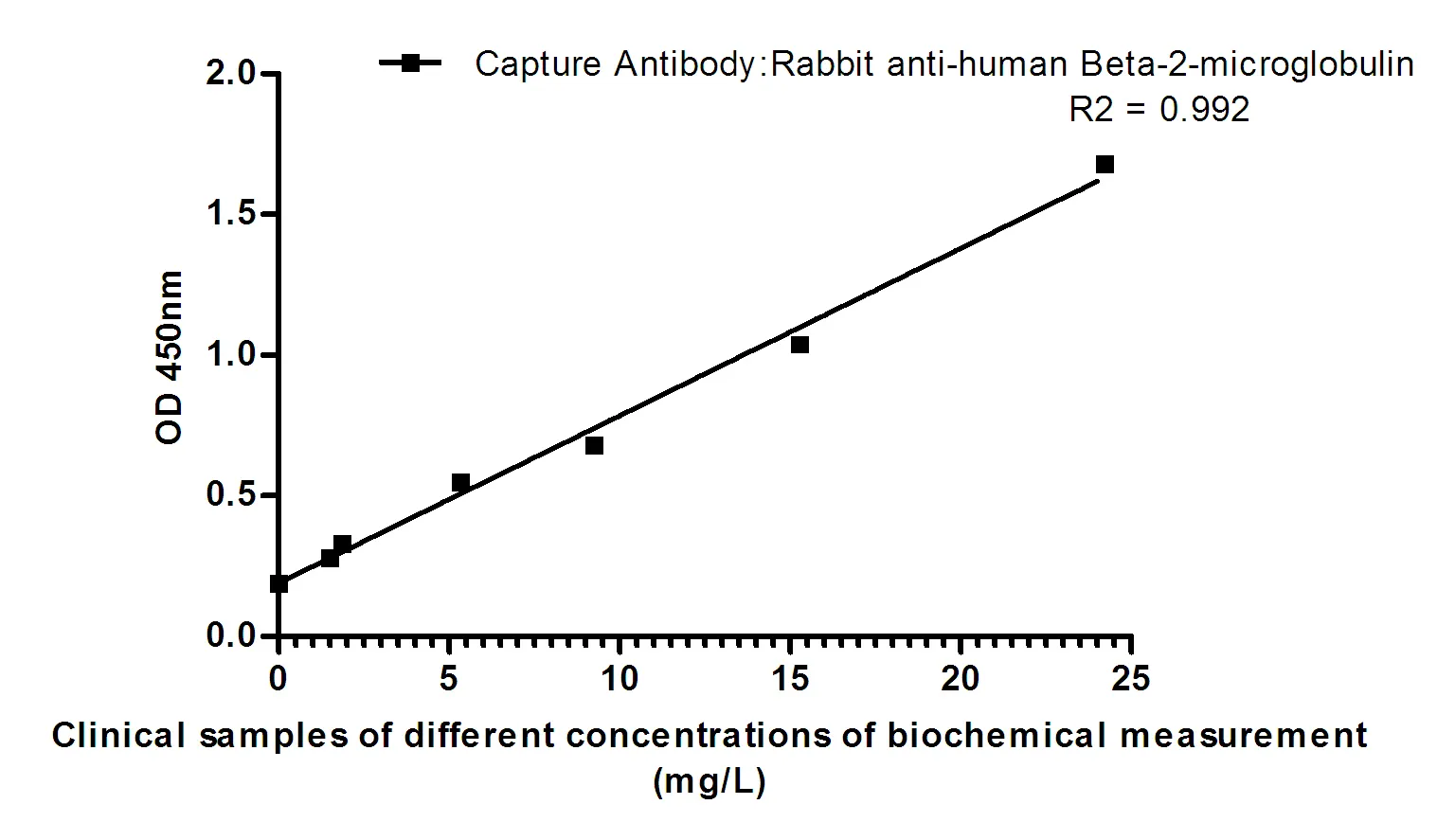
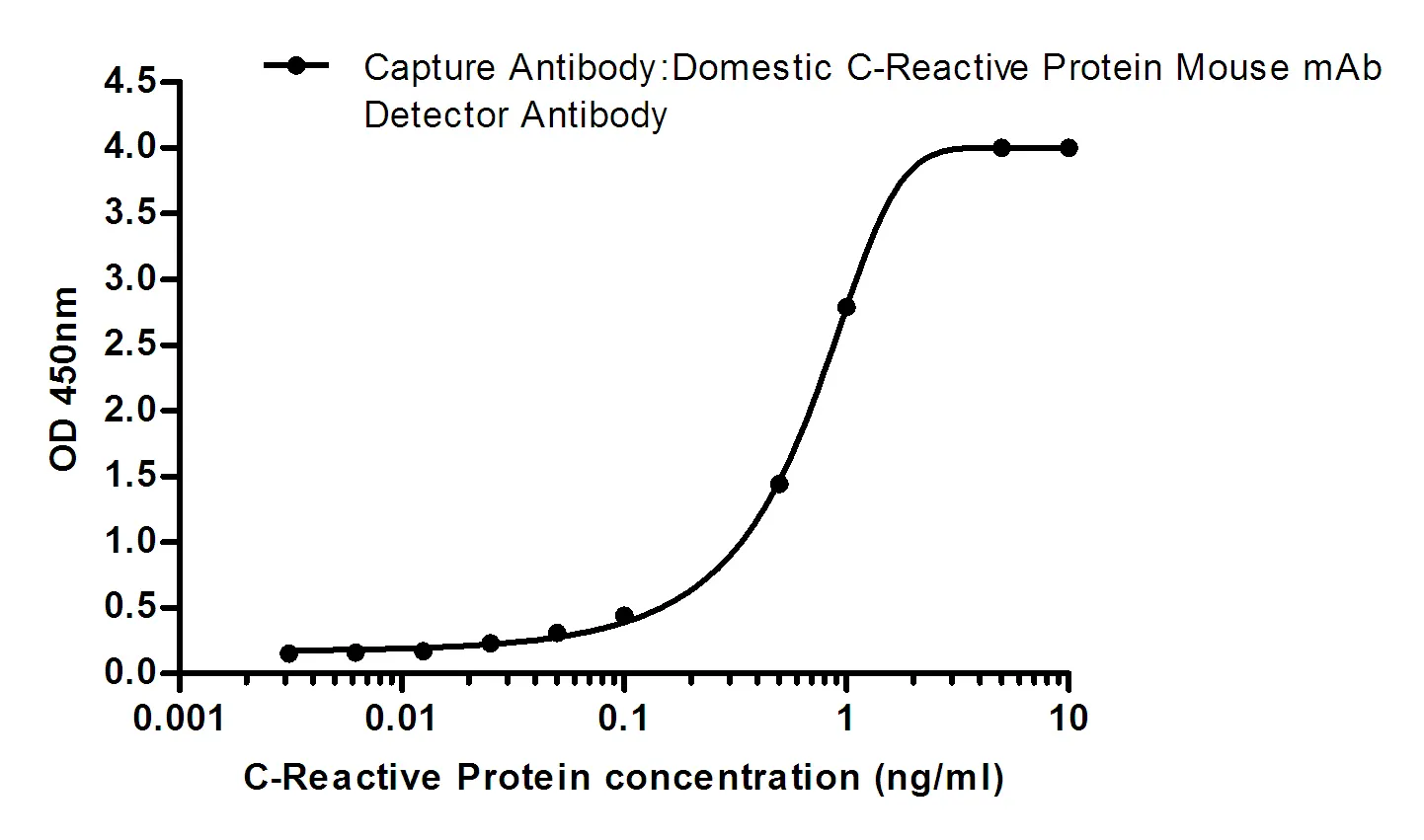
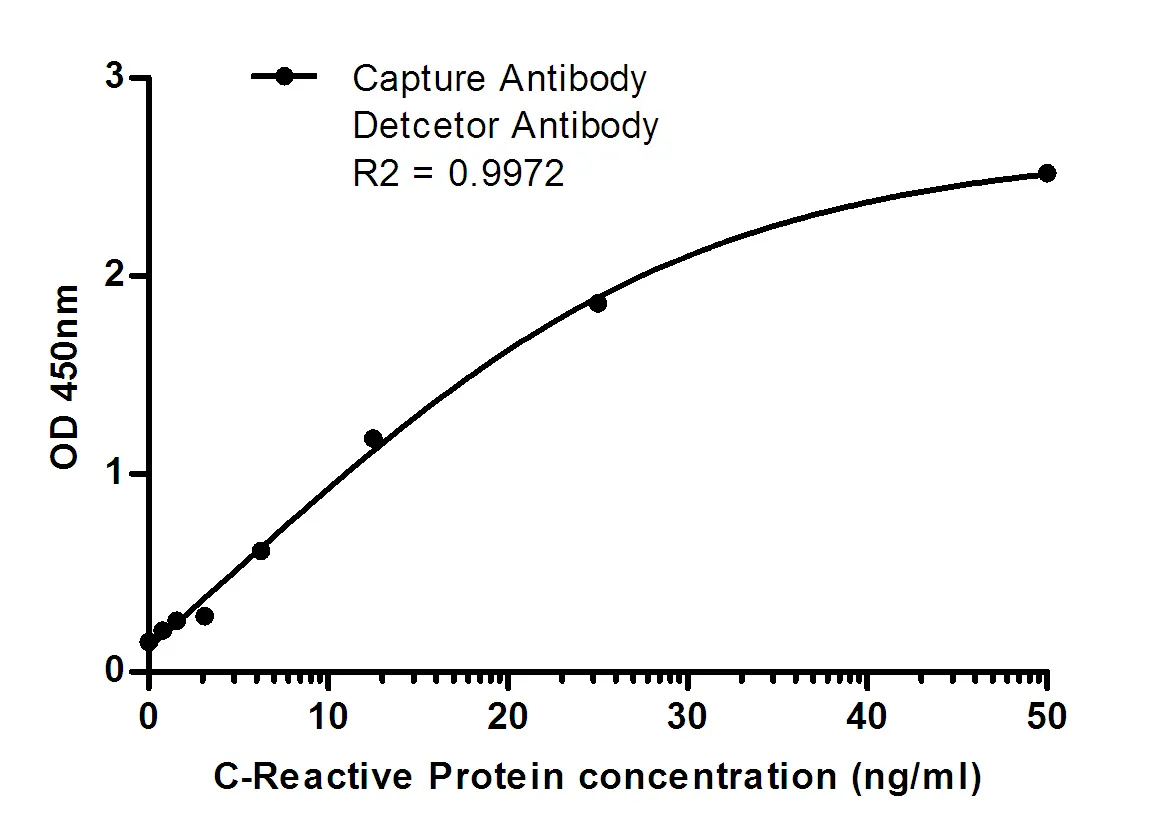
Summary
Performance
Immunogen
Application
Background
Acetylation of lysine, like phosphorylation of serine, threonine or tyrosine, is an important reversible modification controlling protein activity. The conserved amino-terminal domains of the four core histones (H2A, H2B, H3, and H4) contain lysines that are acetylated by histone acetyltransferases (HATs) and deacetylated by histone deacetylases (HDACs). Signaling resulting in acetylation/deacetylation of histones, transcription factors, and other proteins affects a diverse array of cellular processes including chromatin structure and gene activity, cell growth, differentiation, and apoptosis. Recent proteomic surveys suggest that acetylation of lysine residues may be a widespread and important form of posttranslational protein modification that affects thousands of proteins involved in control of cell cycle and metabolism, longevity, actin polymerization, and nuclear transport. The regulation of protein acetylation status is impaired in cancer and polyglutamine diseases , and HDACs have become promising targets for anti-cancer drugs currently in development.
Research Area