KEY FEATURES
| Full Name | Interleukin Enhancer Binding Factor 3 |
|---|---|
| Synonym | DRBF; DRBP76; MMP4; MPHOSPH4; MPP4; NFAT; NF-AT-90; NF90; NFAR1; TCP80; Nuclear Factor Of Activated T-Cells; M-Phase Phosphoprotein 4 |
| Assay Type | Sandwich |
| Reactivity | Human |
| Range | 0.15-10ng/mL |
| Sensitivity | 0.06ng/mL |
| Sample Type | Serum,plasma and other biological fluids |
| Sample Volume | 100μL |
| Detection Wavelength | OD450 |
| Transportation Temperature | 2-8°C |
| Specificity | The kit detected Human ILF3 in the samples and no significant cross-species reactions were found |
| Microplate | 96-wells plate breakable into 12 x 8 wells strip |
TEST PRINCIPLE
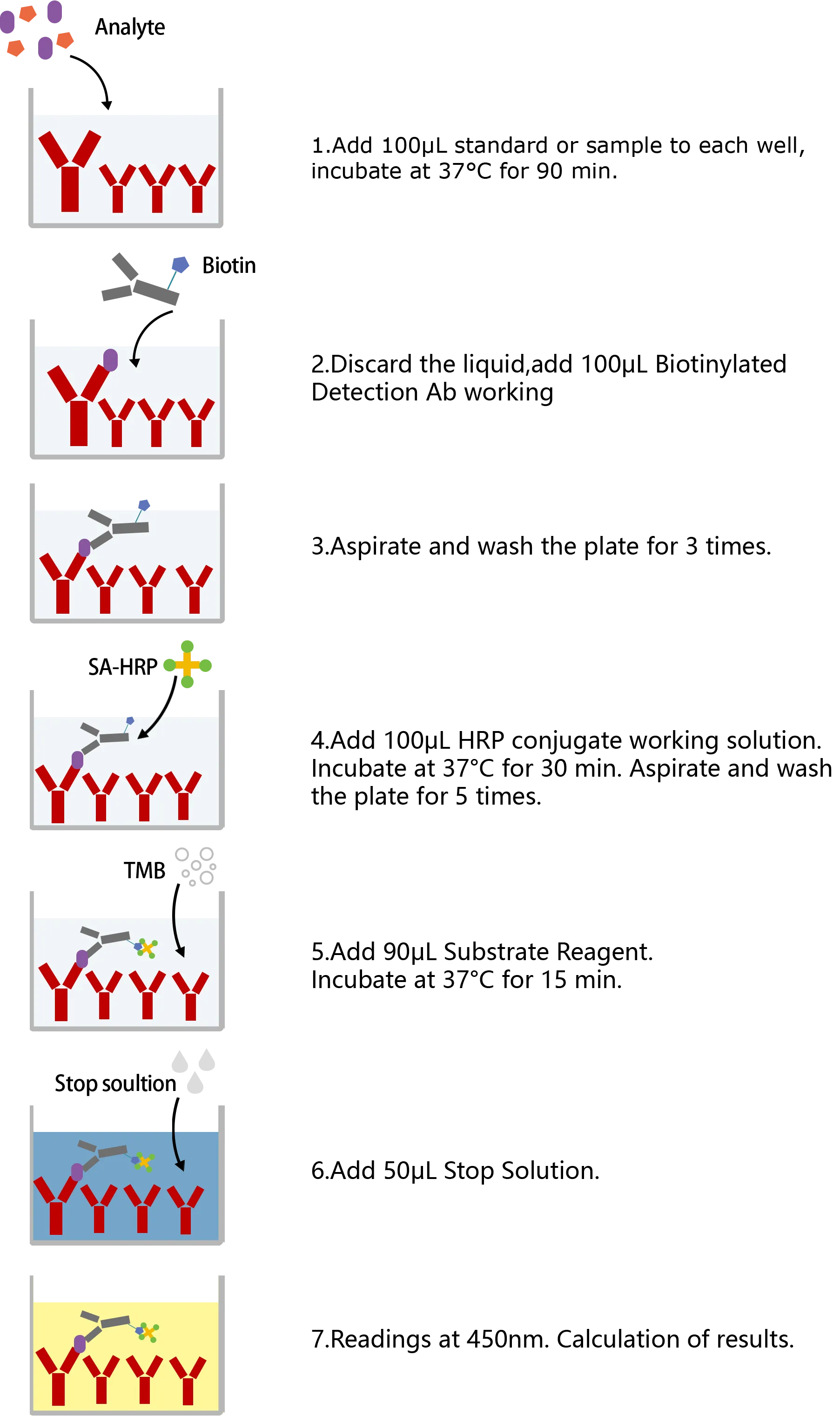
| This ELISA kit uses the Sandwich-ELISA principle. The micro ELISA plate provided in this kit has been pre-coated with an antibody specific to Human ILF3. Standards or samples are added to the micro ELISA plate wells and combined with the specific antibody. Then a biotinylated detection antibody specific for Human ILF3 and Avidin-Horseradish Peroxidase (HRP) conjugate are added successively to each micro plate well and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain Human ILF3, biotinylated detection antibody and Avidin-HRP conjugate will appear blue in color. The enzyme-substrate reaction is terminated by the addition of stop solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The OD value is proportional to the concentration of Human ILF3. You can calculate the concentration of Human ILF3 in the samples by comparing the OD of the samples to the standard curve. |
ELISA KIT COMPONENTS
Upon receipt, unpack promptly and store as recommended in the instructions.
| Components | Specifications | Storage and Notes |
|---|---|---|
| Micro Plate | 96T: 8 wells×12 strips 48T: 8 wells×6 strips | Unopened: -20°C, 12 months Unused: Put it back in the aluminum foil bag and seal it, store it at -20°C. |
| Reference Standard | 96T: 2 vials 48T: 1 vial | Unopened: -20°C, 12 months Please use freshly dissolved standards for each experiment. Discard any unused standards after dissolution. |
| Biotinylated Detection Ab Concentrate (100×) | 96T: 120μL×1 vial 48T: 60μL×1 vial | Unopened: -20°C, 12 months Unused: Please seal the concentrate and store it at -20°C, and discard the working solution. |
| HRP Conjugate Concentrate (100×) | 96T: 120μL×1 vial 48T: 60μL×1 vial | Unopened: -20°C(Protect from light), 12 months Unused: Please seal the concentrate and store it at -20°C, and discard the working solution. |
| Biotinylated Detection Ab Diluent | 14mL×1 | 2-8℃, 12 months |
| HRP Conjugate Diluent | 14mL×1 | 2-8℃, 12 months |
| Reference Standard & Sample Diluent | 20mL×1 | 2-8℃, 12 months |
| Washing Buffer Concentrate (25×) | 30mL×1 | 2-8℃, 12 months |
| Substrate Reagent(TMB) | 10mL×1 | 2-8°C(Protect from light),12 months |
| Stop Solution | 7mL×1 | 2-8°C/Room temperature |
ASSAY PROCEDURES
TYPICAL DATA
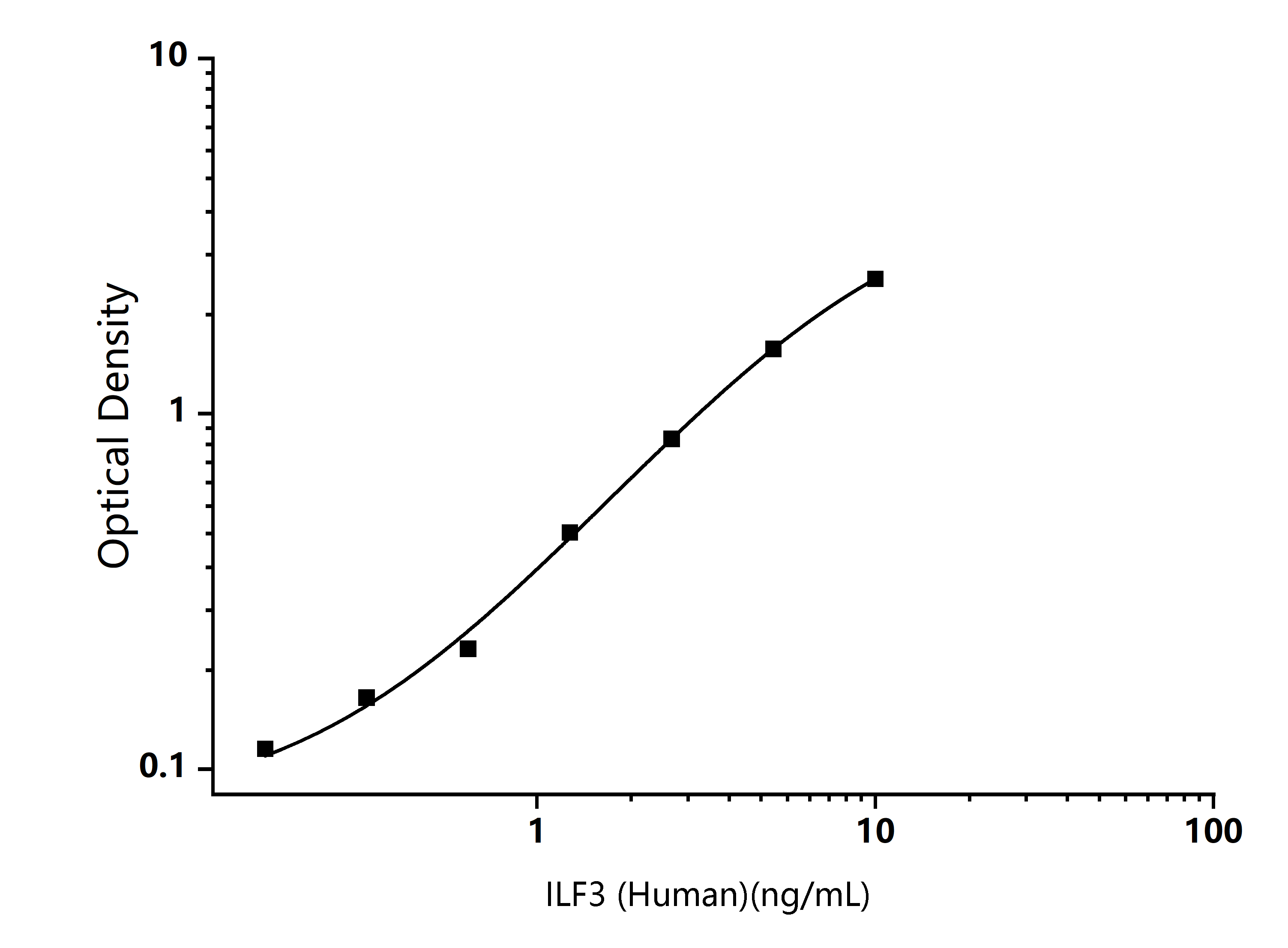
Human ILF3 ELISA Standard Curve
Typical data are for reference only and curves should be replotted for each experiment. The Logistics function is recommended for fitting.
PRECISION
Intra-Assay Precision (Precision within an assay): Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): Three samples of known concentration were tested in twenty separate assays to assess inter-assay precision. Assays were performed by at least three technicians using two lots of components.
| Intra-assay Precision | Inter-assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (ng/mL) | 0.5 | 1.26 | 4.73 | 0.54 | 1.35 | 4.63 |
| Standard deviation | 0.03 | 0.07 | 0.21 | 0.03 | 0.06 | 0.17 |
| CV(%) | 5.37 | 5.81 | 4.46 | 5.71 | 4.78 | 3.61 |
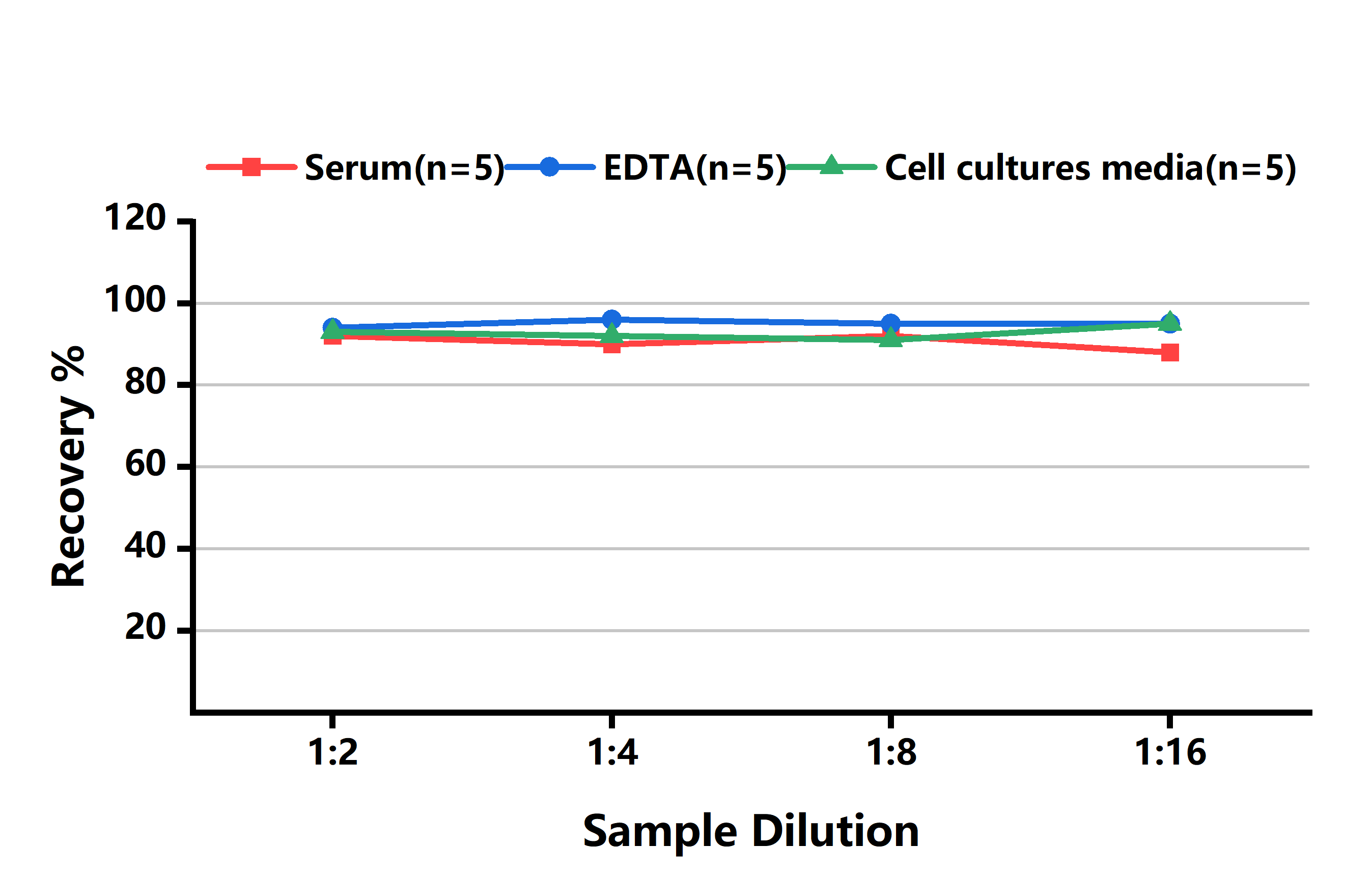
RECOVERY
The recovery of Human ILF3 spiked to three different levels in samples throughout the range of the assay in various matrices was evaluated.
| Sample Type | Range (%) | Average Recovery (%) |
|---|---|---|
| Serum(n=8) | 90-103 | 95 |
| EDTA plasma (n=8) | 85-95 | 90 |
| Cell culture media (n=8) | 89-105 | 96 |
LINEARITY
To assess the linearity of the assay, samples containing and/or spiked with high concentrations of Human ILF3 in various matrices were diluted with the Reference Standard & Sample Diluent to produce samples with values within the dynamic range of the assay.
Products associated with ILF3 ELISA Kits
| XPO5 ELISA Kit | publications with ILF3 and XPO5 |
| PRMT1 ELISA Kit | publications with ILF3 and PRMT1 |
| ZNF346 ELISA Kit | publications with ILF3 and ZNF346 |
| FUS ELISA Kit | publications with ILF3 and FUS |
| DHX9 ELISA Kit | publications with ILF3 and DHX9 |
Diseases associated with ILF3 ELISA Kit
| Neoplasms | publications with ILF3 and Neoplasms |
| Myocardial Infarction | publications with ILF3 and Myocardial Infarction |
| Heart Diseases | publications with ILF3 and Heart Diseases |
| Skin Diseases | publications with ILF3 and Skin Diseases |
| Kidney Diseases | publications with ILF3 and Kidney Diseases |
| Breast Neoplasms | publications with ILF3 and Breast Neoplasms |
| Leukemia | publications with ILF3 and Leukemia |
| Adenocarcinoma | publications with ILF3 and Adenocarcinoma |
| Disease Models, Animal | publications with ILF3 and Disease Models, Animal |
| Endometrial Neoplasms | publications with ILF3 and Endometrial Neoplasms |
Organs/Tissues associated with ILF3 ELISA Kit
| Blood | publications with ILF3 and Blood |
| Brain | publications with ILF3 and Brain |
| Liver | publications with ILF3 and Liver |
| Kidney | publications with ILF3 and Kidney |
| Testis | publications with ILF3 and Testis |
| Spleen | publications with ILF3 and Spleen |
| Lung | publications with ILF3 and Lung |
| Ovary | publications with ILF3 and Ovary |
| Muscle | publications with ILF3 and Muscle |
| Vascular | publications with ILF3 and Vascular |