Literature Sharing: Spatial Resolution of Glioma Subgroups with Functional Tertiary Lymphoid Structures
Background
In the field of glioma treatment, immunotherapy development has long been limited by unclear understanding of the tumor immune microenvironment. As the most aggressive malignant tumor in the central nervous system, gliomas, particularly glioblastoma (GBM), have long been labeled as "immune-cold" tumors. Barriers such as the blood-brain barrier hindering immune cell infiltration, high expression of immune checkpoint molecules like PD-L1 by tumor cells, and enrichment of immunosuppressive cells like M2 macrophages contribute to a clinical response rate of less than 15% for therapies like immune checkpoint inhibitors. Tertiary lymphoid structures (TLS) are ectopic lymphoid tissues spontaneously formed locally in tissues, which have been confirmed as core markers of "immune-hot" tumors in solid tumors, capable of recruiting and activating T cells and B cells to construct sustained anti-tumor immune responses, closely associated with favorable prognosis in melanoma, lung cancer, and other tumor patients. However, whether functional TLS exist in gliomas, their spatial characteristics, and clinical significance have always lacked clear answers. The core breakthrough of the article "Spatial immune profiling defines a subset of human gliomas with functional tertiary lymphoid structures" lies in utilizing the spatial resolution capability of multiplex immunofluorescence mIF technology to identify for the first time a unique subgroup with functional TLS in human gliomas, providing a subversive perspective for glioma immune microenvironment research and immunotherapy target development. Precise identification of TLS faces two major technical bottlenecks: first, TLS definition requires specific spatial arrangement of multi-lineage cells, traditional single-stain immunohistochemistry IHC is prone to errors when inferring from serial sections, while flow cytometry loses spatial information; second, functional TLS require simultaneous detection of cell identity markers and functional markers, which traditional technologies struggle to achieve multi-parameter co-localization at the single-cell level. Addressing these bottlenecks, the research team selected a multiplex immunofluorescence technology (mIF) platform based on tyramide signal amplification TSA technology, which possesses advantages of high sensitivity, simultaneous multi-target detection, and complete preservation of spatial information, enabling hundred-fold signal amplification through horseradish peroxidase HRP-catalyzed covalent binding of fluorophores to detect low-abundance functional markers, and multi-round staining elution cycles can complete detection of 6 to 8 targets on the same FFPE section, perfectly matching the multi-dimensional identification needs of TLS.
Experimental Design
Focusing on precise identification and functional verification of TLS, the study constructed a standardized mIF technical system, with core innovations optimized from three aspects: target combination, staining imaging, and quantitative analysis. The article designed two complementary marker detection channels: one containing CD3, CD4, CD8 (T cell subsets), CD20 (B cells), CD21 (follicular dendritic cells), and DAPI, clarifying the typical TLS morphology of T cells surrounding B cell follicles and CD21⁺ cells forming networks through multi-color fluorescence co-localization, solving the qualitative issue of TLS. The advantage of mIF multi-color overlay avoids cell localization errors in traditional single-stain staining. The other channel contains Ki-67 (proliferation), PAX5 (B cell maturation), IgG (antibody secretion), and CD138 (plasma cell marker), allowing observation of the spatial distribution of PAX5⁺ mature B cells, Ki-67⁺ proliferating cells, and CD138⁺IgG⁺ plasma cells, confirming active immune responses within TLS and completing core verification of functional TLS.
To avoid signal crosstalk and false positives, the study established strict experimental specifications with core optimizations including: epitope stability-adapted staining sequence: staining in the order of CD21, CD20 (stable epitopes) to CD3, CD8 (epitopes prone to degradation), combined with mild antigen retrieval conditions to ensure positive detection rate exceeding 80% after multiple cycles. Fluorescent dye optimization for spectral separation: Opal series dyes with spectral overlap <5% were selected, combined with deconvolution algorithms of multi-spectral imaging systems, resulting in a correlation coefficient r >0.92 between single and multi-stain detection, ensuring staining specificity. Whole-section imaging and precise selection: 20× objective whole-scan avoids omission of small TLS structures, combined with digital pathology software to select target areas, ensuring accuracy of quantitative comparison.
TLS Distribution and Functional Correlation in Perivascular Tumor Microenvironment
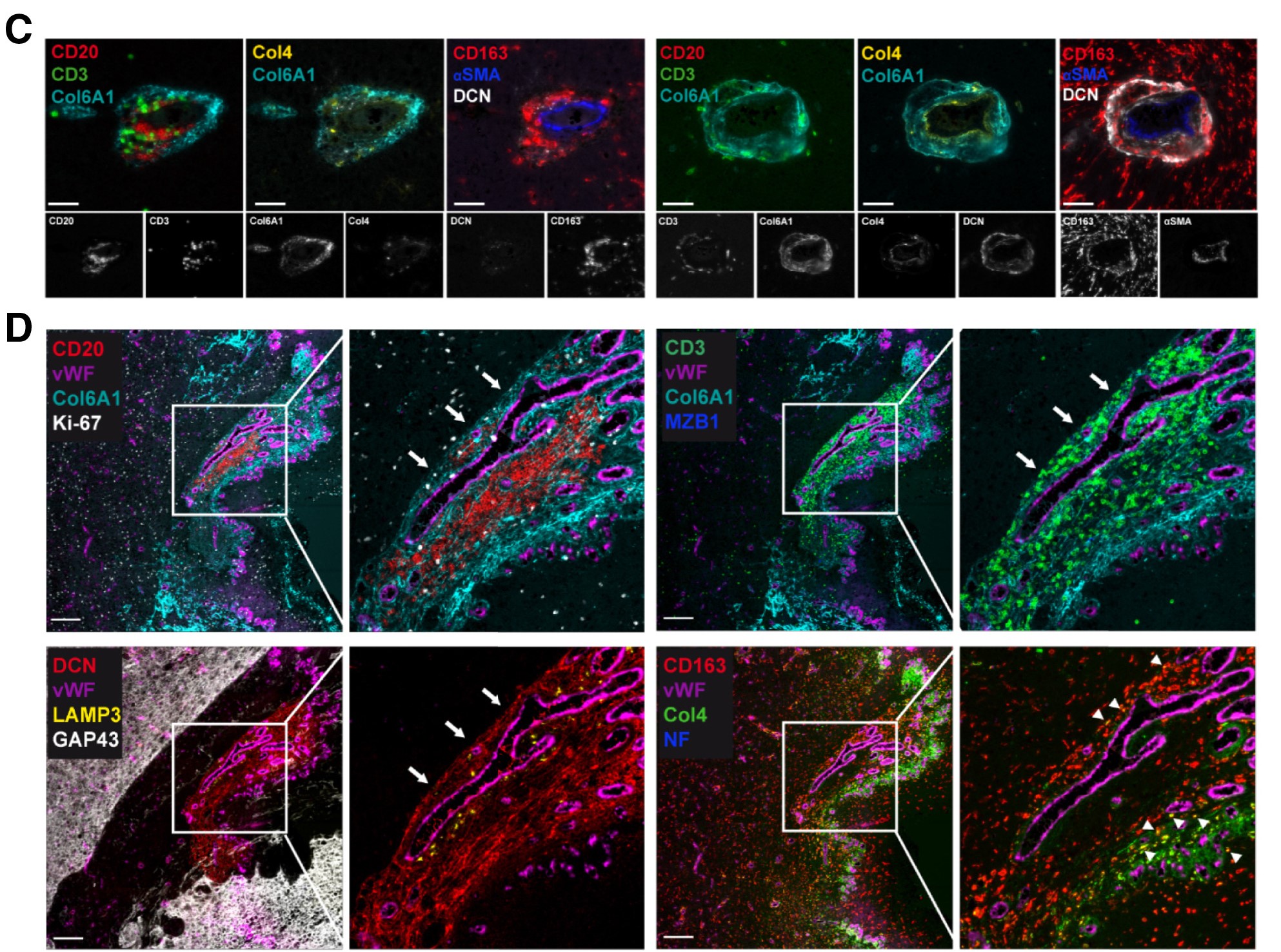
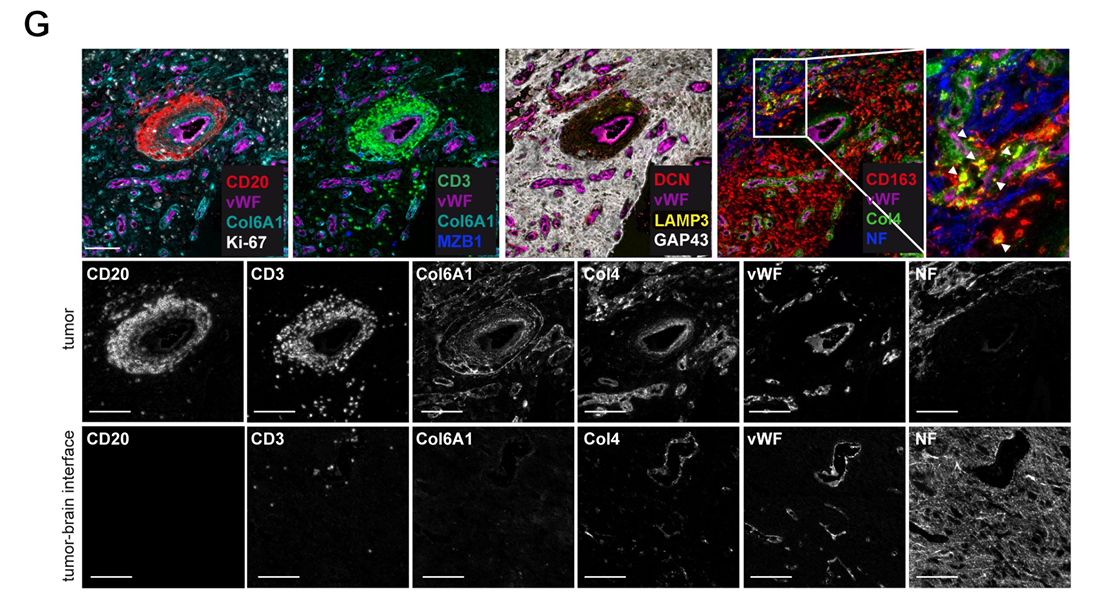
Through multiplex marker immunofluorescence technology, Figure C clearly presents the distribution of CD20⁺B cells and CD3⁺T cells in the perivascular microenvironment, simultaneously displaying the expression localization of extracellular matrix components such as Col6A1, Col4, DCN, αSMA, and CD163, and providing corresponding single-channel images, clarifying the spatial correlation between cells and matrix components. Figure D further clarifies that TLS aggregate in dense extracellular matrix rich in Col6A1⁺ and are adjacent to vWF⁺ blood vessels. High-magnification images show the aggregation morphology of CD20⁺B cells and CD3⁺T cells, while revealing functional active cells such as Ki67⁺ proliferating cells, MZB1⁺ activated differentiating B cells, LAMP3⁺ dendritic cells, and various matrix components within TLS. The discovery of CD163⁺Col4⁺ double-positive macrophages suggests tumor-associated macrophages uptake of Col4 matrix components, and the absence of neurofilament protein expression combined with GAP43 distribution confirms that the TLS is located inside the tumor. A key finding in Figure G is that Col6A1 is not expressed in non-tumor areas.
Structural Functional Characteristics and Maturity Assessment of Glioma-Associated TLS
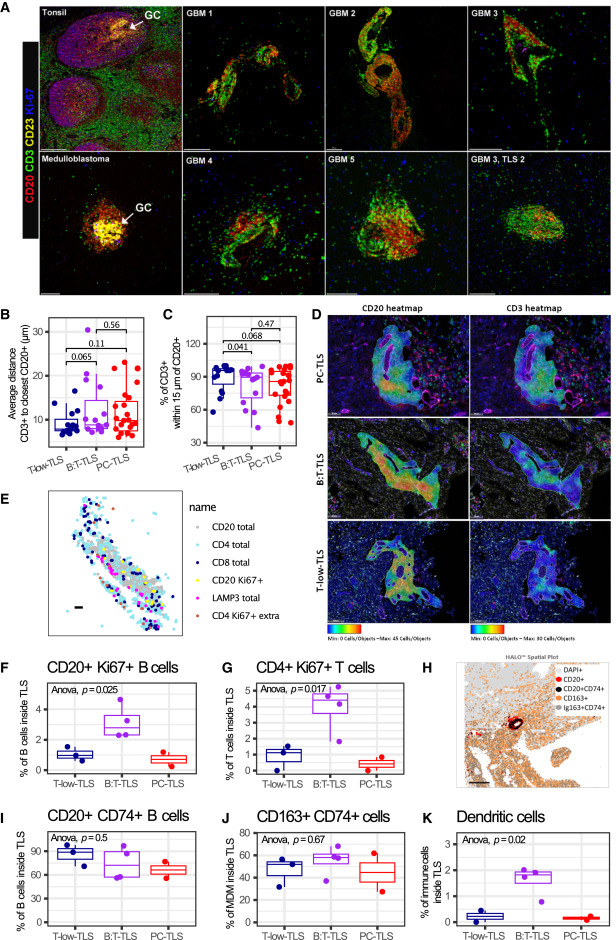
Through multiplex immunofluorescence technology and quantitative analysis, the core characteristics of glioma-associated TLS were revealed. Results showed that all 6 glioma-associated TLS lacked typical germinal centers highly expressing Ki67 and CD23 found in control groups (tonsil, medulloblastoma), and CD23⁺ follicular dendritic cells were not detected. Some subtypes (B:T-TLS, PC-TLS) exhibited T/B cell zonal aggregation characteristics, while T-low-TLS showed more obvious mixed distribution of T/B cells. All TLS subtypes contained proliferative CD20⁺Ki67⁺B cells and CD4⁺Ki67⁺T cells, with B:T-TLS having the highest proliferation ratio. Most B cells and some macrophages within TLS expressed CD74, and the proportion of LAMP3⁺ dendritic cells in B:T-TLS was significantly higher than in other subtypes, along with the presence of follicular helper T cell phenotypes. Some TLS-positive tumors had low and inconsistent transcriptional levels of maturation markers such as AICDA and CR2, indicating limited maturity of glioma-associated TLS, lacking typical germinal center-like reactions.
B Cell Differentiation into Plasma Cells in Glioma-Associated TLS
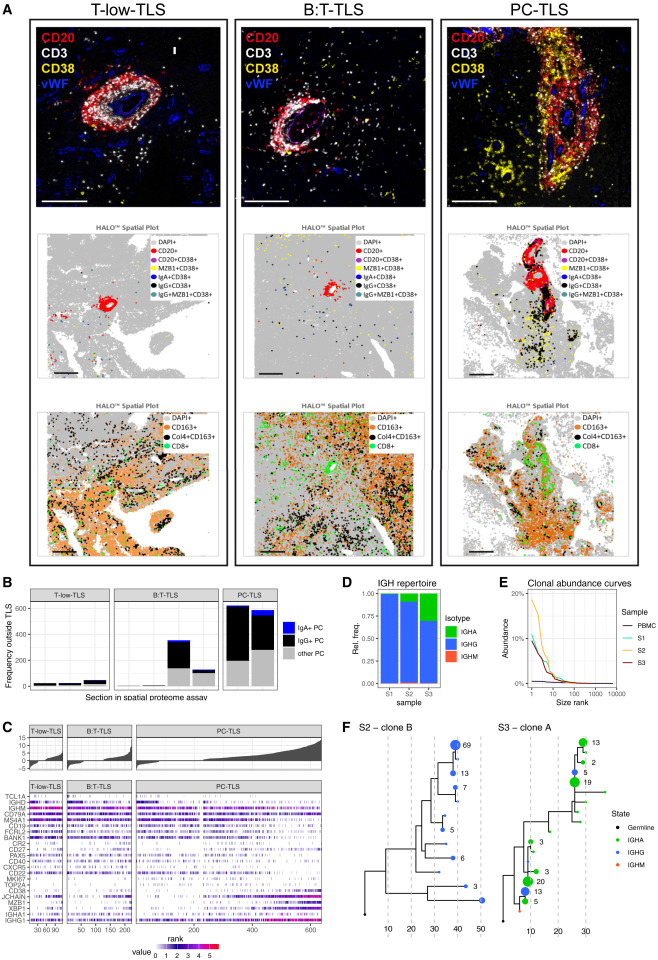
Multiplex mIF detection showed the presence of class-switched plasma cells (IgG⁺CD38⁺MZB1⁺ and IgA⁺CD38⁺MZB1⁺) both inside and outside TLS. The proportion of plasma cells was highest within TLS and their surrounding areas in PC-TLS and some B:T-TLS. Spatial single-cell transcriptome analysis revealed continuous differentiation stages from mature naive B cells (TCL1A, IGHD⁺) to memory-like B cells (IGHG1, CD27⁺) and further to plasmablast-like B cells (CD19, CD38⁺) in all three TLS subtypes. Highly differentiated plasma cells (high expression of IGHG/IGHA and MZB1, low expression of CD19, MS4A1) were mainly enriched in PC-TLS. Immunoglobulin heavy chain sequencing of TLS-positive gliomas showed that the immunoglobulin repertoire was dominated by IgG and supplemented by IgA, consistent with mIF and transcriptome results, and there were expanded clonal families originating from the same VDJ recombination events, which included multiple immunoglobulin subtypes with extensive somatic hypermutations. In summary, the results confirmed the presence of an active differentiation process from naive B cells to plasma cells and memory B cells within glioma tissues, accompanied by affinity maturation and class switch recombination of clonal B cell families.
Conclusion
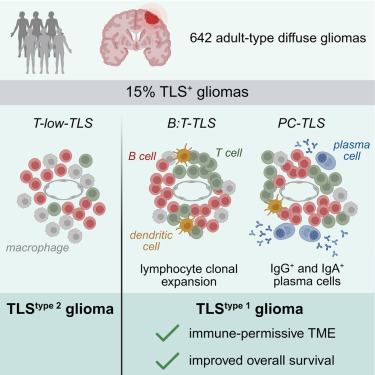
Through multi-modal analysis, this study clearly identified three TLS subtypes with different differentiation states in adult diffuse gliomas, comprehensively evaluated the clinical relevance and maturity-related characteristics of TLS, and identified a TLStype 1 glioma subtype characterized by enrichment of functional TLS (B:T-TLS and PC-TLS), which possesses an immune-permissive microenvironment and longer patient survival. It also proposed an immunofluorescence screening detection method based on pathological tissue sections, which can provide biomarkers for glioma immune typing and immunotherapy response prediction, and offer important reference for patient stratification in GBM immunotherapy trials and development of TLS induction or maturation-targeted therapies.
References
Cakmak P, Lun JH, Singh A, Macas J, Schupp J, Schuck J, Mahmoud Z, Köhler M, Starzetz T, Burger MC, Steidl E, Hasse LM, Hattingen E, Plate KH, Reiss Y, Imkeller K. Spatial immune profiling defines a subset of human gliomas with functional tertiary lymphoid structures. Immunity. 2025 Nov 11;58(11):2847-2863.e8. doi: 10.1016/j.immuni.2025.09.018. Epub 2025 Oct 21. PMID: 41125076.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
