Differences Between Multiplex Immunofluorescence (TSA) and IHC/IF
In the field of in situ protein detection in tissues, immunohistochemistry (IHC), conventional immunofluorescence (IF), and multiplex immunofluorescence (TSA) are the three most widely used technologies. All three are based on specific antigen-antibody binding, but they differ fundamentally in detection throughput, sensitivity, and spatial resolution capabilities, which directly determine their applicable scope in scientific research and clinical settings. This article will systematically analyze the differences between the three from three dimensions: technical core, key performance, and applicable scenarios, providing precise references for experimental design and technology selection.
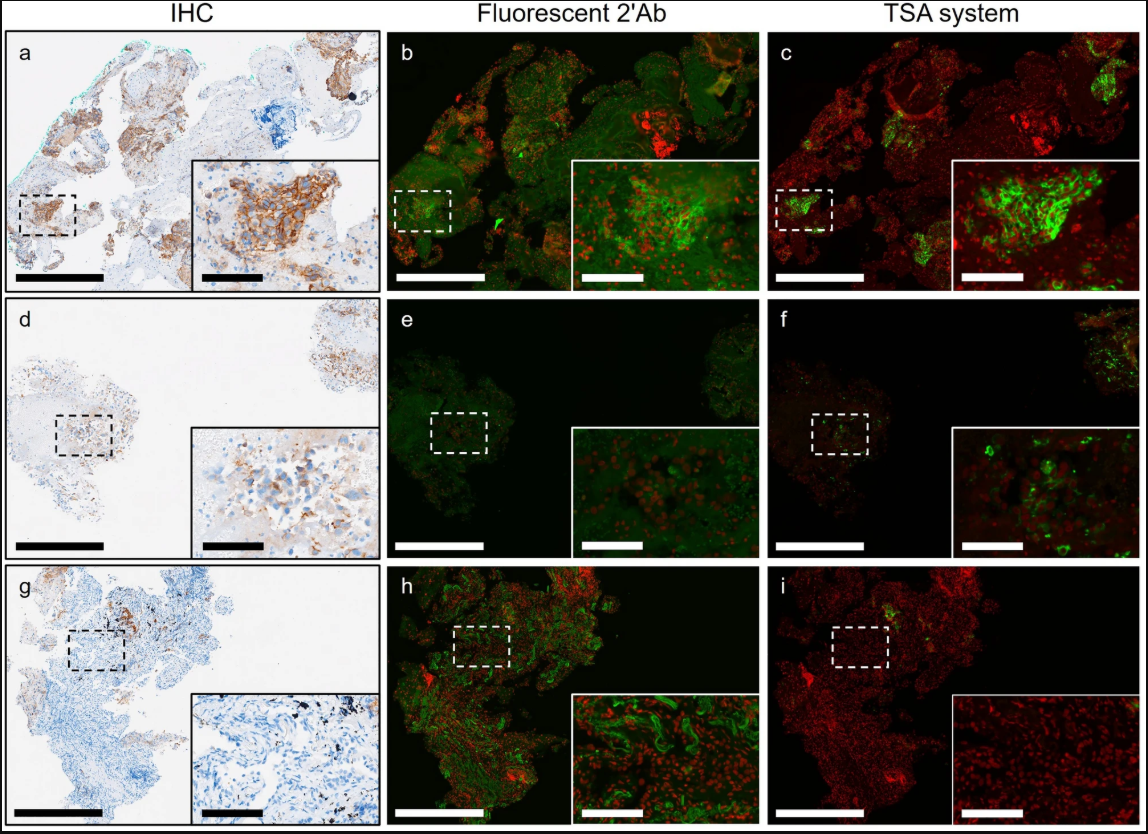
Expression patterns of PD-L1 in IHC, IF, TSA images of NSCLC sections[1]
I. Technical Core Comparison
The core differences among the three technologies stem from variations in "signal generation and amplification mechanisms," which directly lead to distinct detection capabilities.
1. Immunohistochemistry (IHC): "Single-target Basic Tool" with Enzymatic Chromogenic Reaction
The core principle of IHC is "antibody labeling-enzymatic chromogenic reaction": After primary antibody binds to the target protein, secondary antibody conjugated with horseradish peroxidase (HRP) or alkaline phosphatase (AP) catalyzes substrates (such as DAB, AEC) to generate insoluble colored precipitates, which can be directly observed under a light microscope. Signal generation does not require fluorescence excitation, relying on chemical reactions between enzymes and substrates, and has no signal amplification mechanism—signal intensity completely depends on the natural abundance of the target protein and antibody binding efficiency.
Since colored precipitates cannot be specifically removed, only one target can be detected at a time on a single slice. If multi-target relationships need to be analyzed, multiple serial sections must be repeatedly prepared and indirectly associated through image registration, which can easily lead to data bias due to section differences.
2. Conventional Immunofluorescence (IF): "Limited Multi-target Tool" with Direct Fluorescent Labeling
IF replaces IHC's "enzymatic chromogenic reaction" with "direct fluorescent molecule conjugation": Secondary antibodies are directly linked to fluorophores (such as FITC, Cy3, Cy5), generating fluorescent signals upon irradiation with specific wavelength excitation light to achieve target localization. To break through the single-target limitation, conventional IF can use different fluorophores to label antibodies for multi-target detection, but it is limited by two core bottlenecks:
Fluorescence spectral overlap: The excitation/emission spectra of different fluorophores tend to overlap (e.g., FITC and Cy3 have a spectral overlap rate of 30%), limiting stable detection to a maximum of 3-4 targets on a single slice, beyond which severe signal crosstalk occurs.
No signal amplification: Similar to IHC, IF signal intensity depends on target abundance, making low-abundance proteins (such as signaling pathway kinases, trace tumor markers) easily masked by background fluorescence, limiting detection sensitivity.
3. Multiplex Immunofluorescence (TSA): "High-throughput Precision Tool" with Signal Cascade Amplification
TSA technology, fully known as Tyramide Signal Amplification, is a revolutionary technology based on IF that introduces "enzymatic signal cascade amplification + cyclic labeling". Its core principle consists of two steps:
Signal amplification: After the primary antibody binds to the target, the secondary antibody conjugated with HRP catalyzes the oxidative polymerization of tyramide molecules carrying fluorophores, causing numerous fluorescent molecules to covalently bind to proteins around the target, forming a "target-antibody-HRP-fluorescent polymer" complex. Signal intensity is 50-100 times higher than conventional IF.
Cyclic labeling: After completing the first round of detection, HRP activity is quenched and primary/secondary antibodies are stripped using gentle chemical methods, leaving only covalently bound fluorescent signals. Then, the "primary antibody binding-TSA amplification" process is repeated with fluorophores of different spectra to achieve multiple rounds of target labeling.
This combination of "signal amplification + cyclic labeling" completely breaks through the target number and sensitivity limitations of conventional IF, enabling easy detection of 5-10 colors or more targets on a single slice, with even low-abundance protein signals clearly distinguishable.
II. Key Performance Comparison
Performance Dimension | Immunohistochemistry (IHC) | Conventional Immunofluorescence (IF) | Multiplex Immunofluorescence (TSA) |
|---|---|---|---|
Antibody Selection | Only one primary antibody can be used per section | Different primary antibodies from different species, and secondary antibodies from different species conjugated with different fluorophores | Can use different primary antibodies from the same species with HRP-labeled secondary antibody from the same species |
Detection Sensitivity | Low (no signal amplification, only detects high-abundance proteins) | Medium (relies on fluorescence intensity, low-abundance proteins easily missed) | Extremely high (50-100 times signal amplification, can detect very low-abundance targets) |
Spatial Resolution | Low | Medium | High |
Background Noise | Medium (non-specific enzymatic reactions easily produce precipitates) | High (tissue autofluorescence + non-specific antibody binding) | Low (strong targeting of signal amplification, cyclic quenching reduces residues) |
Quantification Capability | Qualitative/Semi-quantitative | Semi-quantitative | Precise quantification (stable fluorescent signals, compatible with AI image analysis) |
Operation Difficulty | Relatively simple, standardized | Relatively simple, standardized | Multiple steps, requires careful optimization |
Experimental Cost | Low | Primary and secondary antibodies need to be from different species, high antibody cost Mixed staining is not conducive to optimizing experimental conditions, easily wastes sections, increasing experimental costs | High antibody applicability, cost-saving Multi-round staining experiments with removable antibodies, convenient for adjusting experimental protocols at any time |
Core Conclusion: IHC is a "basic qualitative tool", IF is a "limited multi-target tool", and TSA is a "high-throughput precision tool". The three are not alternatives but hierarchical choices for different needs—when research focuses on a single high-abundance target, IHC is economical and efficient. When co-localization of up to 3 targets is needed, IF is easy to operate. When complex molecular networks (such as multi-cell interactions in tumor microenvironment) need to be analyzed, TSA is the only feasible technology.
III. Application Scenario Comparison
Differences in technical performance directly determine their applicable scenarios:
1. Immunohistochemistry (IHC): "Standard Screening Tool" for Clinical Pathology Diagnosis
The core advantages of IHC are simple operation, low cost, stable results, and long-term preservation of colored precipitates, making it an "entry-level tool" for clinical pathology diagnosis, mainly used for:
Basic tumor classification: such as marking epithelial-derived tumors with CK (cytokeratin) and mesenchymal-derived tumors with Vimentin.
Single target screening: such as preliminary screening of HER-2 protein expression in breast cancer and qualitative detection of EGFR protein expression in lung cancer.
Rapid verification in basic research: such as verifying the expression site of specific proteins in tissues.
2. Conventional Immunofluorescence (IF): "Simple Multi-target Tool" for Basic Research
IF does not require enzymatic reactions, can achieve rapid localization of targets, and can simultaneously observe the spatial relationship of 2-3 targets, making it suitable for "simple molecular interaction" analysis in basic research. Main scenarios include:
Protein co-localization verification: such as labeling protein A with FITC and protein B with Cy3 to observe whether they overlap in the cytoplasm.
Preliminary distinction of cell subsets: such as double-labeling CD3 (T cells) and CD20 (B cells) in immune tissues to observe distribution differences between the two cell types.
Live cell dynamic observation: such as labeling cytoskeletal proteins (microtubules, microfilaments) to track structural changes during cell division in real-time.
3. Multiplex Immunofluorescence (TSA): "Core Tool" for Complex System Analysis
The high-throughput and high-sensitivity advantages of TSA make it a core technology for analyzing "complex molecular networks" and "fine spatial relationships", particularly suitable for complex scenarios such as tumor microenvironment and neuroscience. Main applications include:
Panoramic analysis of tumor microenvironment: Simultaneously label tumor cells, immune cells, immune checkpoints, and blood vessels, quantifying the spatial distribution and interaction relationships of various cell subsets.
Neuroscience research: Label multiple protein markers on neurons and synapses to deeply study the structure and function of the nervous system.
Immunotherapy development: By detecting multiple receptors and molecular markers on the surface of immune cells, in-depth study of immune cell differentiation, function, and their interactions can reveal the operating mechanisms of the immune system.
Support for spatial omics: TSA dyes can provide spatial resolution information of molecular expression in cells and tissues. Spatial omics combines genomics, proteomics, and other omics technologies to analyze biomolecules while preserving the original spatial structure of samples.
AI-assisted analysis: High-resolution, low-background multicolor data, compatible with AI algorithms to achieve single-cell segmentation, target quantification, and automated diagnostic model construction.
IV. Selection Recommendations: Three Steps to Determine the Optimal Technical Solution
Combining the above differences, in practical work, you can select technologies through a three-step method of "clarifying needs - matching performance - balancing costs":
Step 1: Clarify Core Needs — Determine whether it is single-target qualitative analysis, 2-3 target co-localization, or network analysis of more than 3 targets.
Step 2: Match Technical Performance — Choose IHC for high-abundance single targets, conventional IF for 2-3 targets, and TSA for complex multi-targets or low-abundance targets.
Step 3: Balance Cost and Efficiency — IHC has the lowest cost and shortest cycle; conventional IF has moderate cost and shorter cycle; TSA has higher cost and longer cycle, but the data value far exceeds the cost input.
Enkilife not only provides customers with a complete set of TSA multiplex labeling kits, but also offers various TSA specialty technical services, including IF fluorescence staining, fluorescence panoramic scanning, ultra-multiplex staining, and pathological analysis (5 markers and below).
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
For details, please check TSA mIHC Kit
References
1. Huang HN, Kuo CW, Hung YL, Yang CH, Hsieh YH, Lin YC, Chang MD, Lin YY, Ko JC. Optimizing immunofluorescence with high-dynamic-range imaging to enhance PD-L1 expression evaluation for 3D pathology assessment from NSCLC tumor tissue. Sci Rep. 2024 Jul 2;14(1):15176. doi: 10.1038/s41598-024-65187-x. PMID: 38956114; PMCID: PMC11219731.
