mIHC-TSA Experiment: Key Technical Points and Typical Q&A
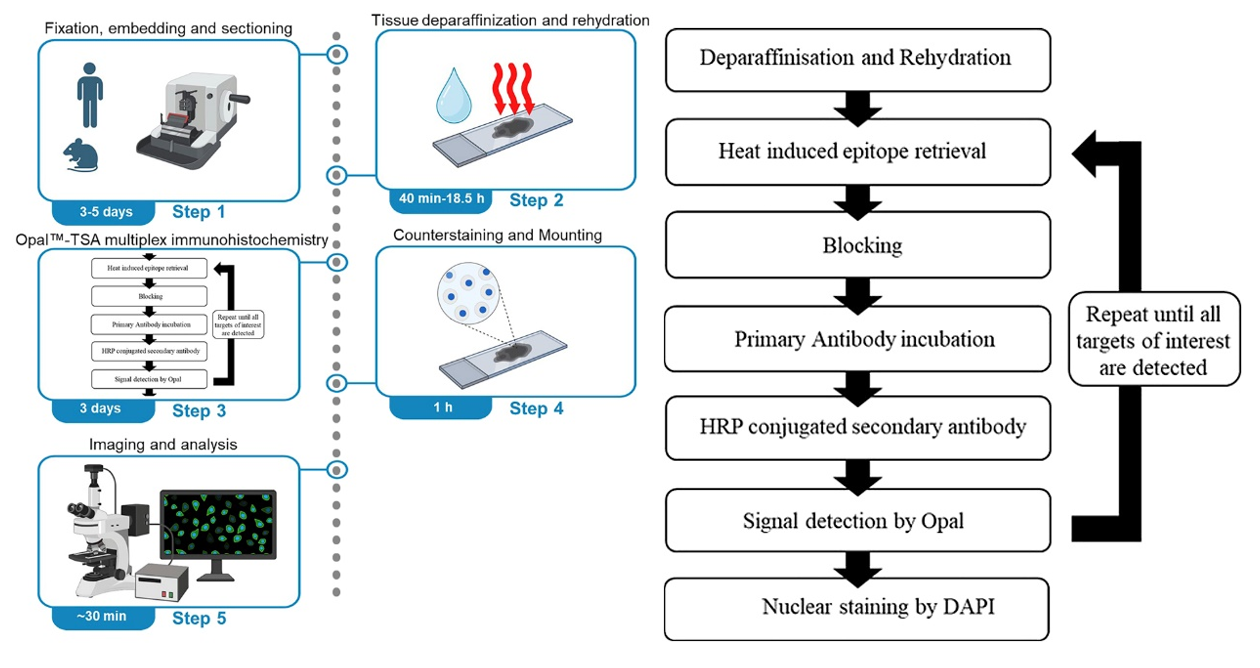 I. Key Technical Points
I. Key Technical Points
1. Antigen Retrieval: Antigen retrieval typically uses 1× citric acid (pH 6.0) as the retrieval solution, with high-temperature high-pressure retrieval. Place the sections in a pressure cooker, add an appropriate amount of retrieval solution, close the pressure cooker, and time for 2 minutes after steam appears. Cool to room temperature to complete retrieval. [For targets with weak expression, EDTA (pH 9.0) can be used as the retrieval solution. For tissues prone to detachment such as bone or brain tissue, microwave retrieval is recommended, with retrieval temperature controlled at around 80℃, and 2× citric acid (pH 6.0) can be used as the retrieval solution.]
2. Endogenous Enzyme Blocking: Prepare 3% H2O2 solution with pure water, place the sections in the solution, and incubate at room temperature for 20 minutes. For tissues prone to detachment, appropriately reduce the H2O2 concentration and incubation time. If samples contain hemoglobin (such as liver or spleen), extend the incubation time as needed to avoid autofluorescence caused by hemoglobin.
3. Antibody Selection and Dilution: Prioritize the use of monoclonal antibodies and avoid polyclonal antibodies. For mouse samples, try to avoid choosing mouse-derived primary antibodies. If mouse primary antibodies are selected, the secondary antibody will bind not only to the primary antibody but also to endogenous IgG in the tissue, resulting in non-specific staining. The dilution ratio of the primary antibody needs to be determined through preliminary experiments. Excessively high concentration may lead to non-specific binding, while excessively low concentration may result in weak signals. It is recommended to set up concentration gradients to screen for optimal conditions.
4. TSA Dye Usage Specifications: TSA dyes are photosensitive substances, and working solutions need to be freshly prepared. For low-expression targets, choose dyes with high fluorescence intensity and strong anti-fade properties (such as Cy5, AF594) to enhance detection rate using the TSA signal amplification effect. For high-expression targets, choose dyes with moderate fluorescence intensity (such as FITC, Cy3) to avoid signal saturation masking details.
5. Avoid Spectral Overlap: Select dye combinations with emission wavelengths spaced ≥50nm apart, for example: DAPI (461nm) + AF488 (519nm) + Cy3 (570nm) + Cy5 (670nm), which can effectively reduce cross-talk. Avoid using FITC (520nm) and AF488 (519nm), or Cy3 (570nm) and AF594 (617nm) simultaneously.
6. Compatibility with Imaging Equipment: For fluorescent TSA, confirm whether the fluorescence channels are compatible with the wavelength of the laboratory microscope. When using an ordinary fluorescence microscope, prioritize visible light region dyes (FITC, Cy3, DAPI) and avoid far-infrared dyes (Cy5). When using a confocal or multispectral microscope, far-infrared dyes (Cy5, AF647) can be used, and multispectral separation technology can be utilized to further reduce cross-talk.
7. Antibody Elution Efficiency: If some antibodies have high titer and strong affinity and are not easily eluted thoroughly, increase the number of elution cycles. Antibody elution solution has high fluidity; if the slides are not placed horizontally, the reagent may flow out of the circle, affecting elution efficiency. Keep slices flat during operation. For samples prone to detachment, reduce the elution solution temperature or shorten the incubation time.
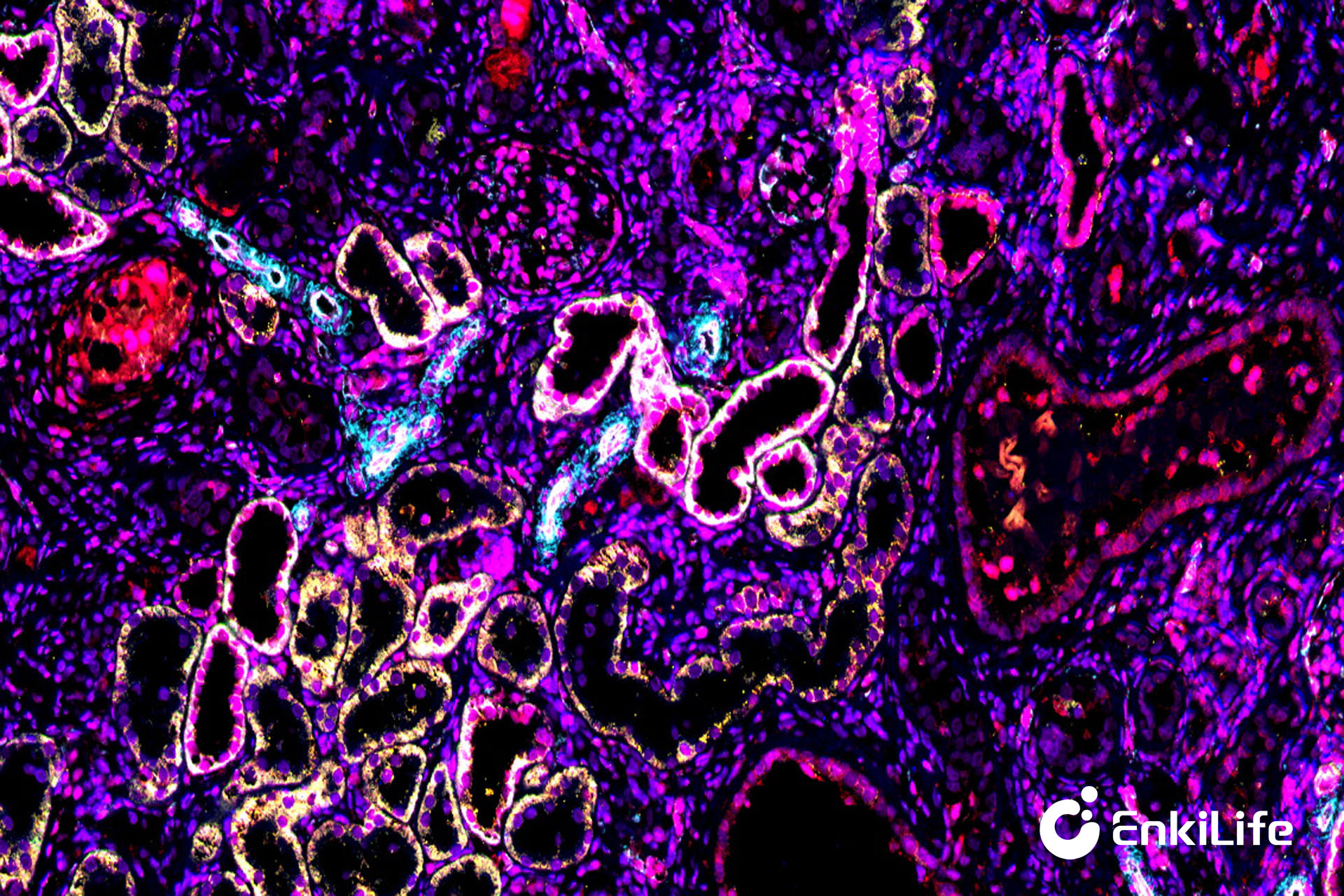
20× Rat Kidney 6-marker 7-color Result
II. Common FAQ
1. Can frozen floating sections be used for TSA experiments?
Answer: The TSA kit is not suitable for floating sections. Because floating sections are too thick, which is not conducive to reagent penetration. Floating sections may change shape during staining, such as curling or wrinkling.
2. How to choose antibodies for paraffin section mIHC?
Answer: Try to use monoclonal antibodies, with antibody applications selected as IHC/mIHC/IHC-P, and prioritize antibodies that have been verified by knockout experiments.
3. Why does non-specific binding occur?
Answer: a. If polyclonal antibodies are used, they are prone to non-specific binding, which can be improved by switching to monoclonal antibodies or reducing concentration and retrieval intensity. b. If signal amplification is too strong, reduce the dye reaction time or concentration. c. If the primary antibody concentration is too high or antigen retrieval is excessive, use a lower antibody dilution ratio or reduce antigen retrieval intensity.
4. How to avoid section detachment?
Answer: a. If slides are not treated with anti-detachment, use anti-detachment slides or treat the slides with anti-detachment. b. If tissue fixation is incomplete or dehydration is insufficient, re-optimize the sample pretreatment methods, such as extending fixation and dehydration time. c. If excessive thermal antigen retrieval is applied, use gentle microwave retrieval with temperature controlled at around 80℃ or lower, and 2× citric acid (pH 6.0) can be used as the retrieval solution. d. If the elution solution temperature is too high, reduce the elution solution temperature or shorten the incubation time.
5. Why does cross-talk occur?
Answer: a. It may be related to the filter bandwidth of the imaging equipment; try to use filters with narrow wavelength bandwidth. b. It may be related to incomplete elution of the previous round of antibodies; for antibodies with high affinity such as CK, extend the antibody elution conditions (increase elution temperature and time, etc.). c. It may be related to signal imbalance, such as adjacent channel dyes with one being too strong and the other too weak, causing spillover of the strong signal. d. Other possible reasons include mixing up antibodies, forgetting to elute/retrieve in the next round, etc.
6. For mouse samples, can mouse-derived primary antibodies be selected?
Answer: This method is generally not recommended, because if the primary antibody is derived from mouse, the secondary antibody also needs to be anti-mouse, which may cross-react with IgG present in the sample itself, leading to non-specific binding. If it is necessary to use a primary antibody from the same species as the sample, it is recommended to perform a negative control experiment by adding only the secondary antibody without the primary antibody to detect whether non-specific signals are generated.
7. How to choose the order of markers/antibodies for multiplex staining?
Answer: Place antibodies that are difficult to elute in the last round to avoid cross-talk. Alternate between rabbit and mouse primary antibodies. Place markers that are difficult to detect in the earlier rounds. For low-expression targets, choose dyes with high fluorescence intensity and strong anti-fade properties and place them in the earlier rounds. For high-expression targets, choose dyes with moderate fluorescence intensity to avoid signal saturation masking details. If two proteins are co-expressed, it is recommended to choose dyes with large wavelength differences.
8. Can traditional fluorescent staining be used in combination with TSA multiplex immunofluorescence?
Answer: Yes, but it is recommended to arrange traditional fluorescent staining in the last round. This is because during the antibody elution process of the TSA method, both the primary and secondary antibody conjugates are eluted simultaneously.
9. Is it necessary to avoid light during the experimental operation? How long can stained slides be stored?
Answer: The fluorescent dyes of the Enklife TSA kits have excellent anti-fade properties, so there is no need to avoid light under fluorescent lamps, and no need to operate in a dark environment during use. However, please avoid direct exposure of the kit to sunlight. Stained slides can usually be stored at 4℃ for 6 to 12 months.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
