Literature Review: Practical Advances in Multiplex Immunohistochemistry and Immunofluorescence Techniques
1. Research Background
In recent years, the analysis of the complex immune landscape of the tumor microenvironment (TME) has become the core of precision oncology research. However, routine clinical practice still relies on H&E staining and single-marker chromogenic IHC detection, which has limitations such as tissue waste, inability to simultaneously evaluate multiple markers, and unsuitability for quantitative and spatial analysis, making it difficult to meet the needs of tumor microenvironment research and immunotherapy response prediction. The cellular abundance and spatial arrangement of the tumor microenvironment affect tumor progression and treatment response. When using single IHC to evaluate PD-L1 expression to predict immunotherapy response, there are problems such as poor reproducibility, inability to clearly identify the cell type of expression, and difficulty in quantifying spatial arrangement. Multiplex staining, which can simultaneously target multiple proteins through fluorescent or chromogenic staining, can characterize the co-expression profile and spatial arrangement of markers, showing superior performance to multiple detection methods in predicting immunotherapy response. It has wide application value in tumor microenvironment research, spatial heterogeneity analysis of biomarkers, solving the problem of multi-marker detection in small samples, and patient stratification in clinical trials. With continuous optimization and decreasing implementation difficulty, it is more suitable for routine clinical use. "Multiplex Immunohistochemistry and Immunofluorescence: A Practical Update for Pathologists" is a review article for pathologists, focusing on multiplex immunohistochemistry (mIHC) and multiplex immunofluorescence (mIF) techniques. By simultaneously detecting multiple biomarkers on the same section, it greatly improves the information content and accuracy of pathological diagnosis. Currently, the technical approaches to achieve multiplex detection mainly include multiplex IHC/immunofluorescence based on TSA and other methods, mass spectrometry imaging and digital spatial profiling (DSP), and virtual multiplex staining achieved through consecutive section registration. Although these techniques still face challenges in standardized procedures and data analysis, with the maturity of digital pathology technology, multiplex staining has gradually gained feasibility for clinical translation and is expected to become a routine method for guiding tumor immunotherapy decisions in the near future.
2. Conventional Single and Dual Tissue Staining
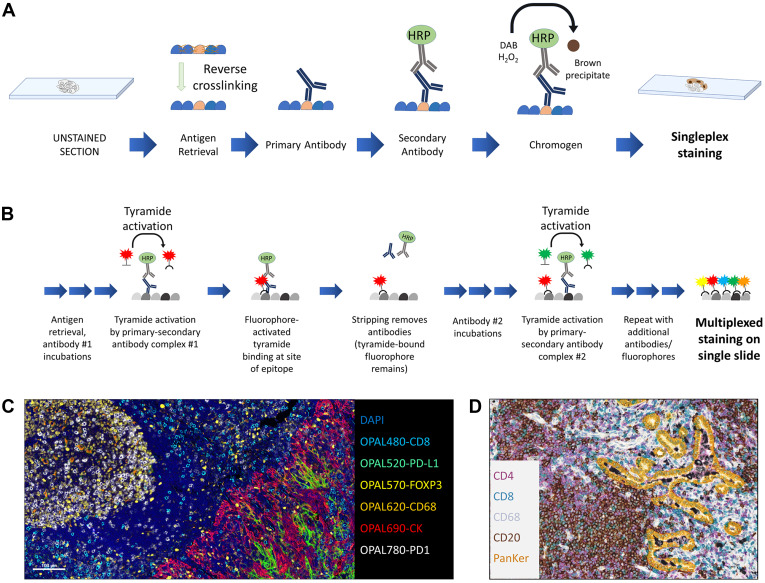
Single Chromogenic Immunohistochemistry
Single chromogenic IHC is used to detect a single antigen in tissue with a single color under bright-field microscopy. Its principle is to use direct or indirect enzyme-linked antibody strategies to develop color at the antigen location and display the tissue background with counterstaining to determine the localization and expression of the target antigen.
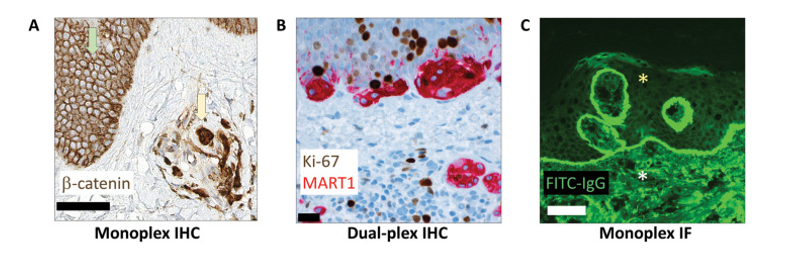
Traditionally, single IHC has been the preferred method in diagnostic pathology due to its mature technology, low cost, simple operation, short turnaround time, permanent archiving of stained slides, and interpretable morphology under light microscopy. It also has well-established clinical validation guidelines, and some markers can be quantitatively analyzed with image algorithms. However, single IHC still has obvious limitations, including mainly relying on pathologists' semi-quantitative manual interpretation, which is prone to inter-observer variability. Differences in experimental procedures between different laboratories also affect the reproducibility of results. Additionally, chromogenic staining has a narrow linear dynamic range, easily leading to signal saturation, which makes it difficult to cover the wide dynamic range of protein expression in biological samples. Therefore, it has deficiencies in clinical scenarios requiring accurate differentiation of low expression levels or strict quantification. Furthermore, since only one marker can be detected at a time, when multiple markers are needed for combined phenotypic analysis, it often relies on comparison of consecutive sections, which may lead to difficulties in accurate spatial localization and co-expression analysis due to inconsistent cell distribution or tissue loss. To address these challenges, many clinical laboratories have gradually carried out dual marker detection, and some centers have begun to use commercial platforms for multiplex bright-field immunostaining to achieve simultaneous analysis of more targets while retaining morphological background.
Dual Chromogenic Immunohistochemistry
Dual chromogenic immunohistochemistry enables visual co-evaluation by staining two different markers on the same tissue section, usually marking one target with DAB and another with naphthol/fast red, etc. Routine applications include Ki-67/MART1 dual staining to evaluate proliferation index in melanocytic lesions, CD3/CD20 dual staining to compare T/B cell populations in immune infiltration, AMACR/p63/high molecular weight keratin in prostate biopsy to distinguish cancer from benign prostate glands, and p40/napsin A or TTF1/CK5/6 to differentiate lung squamous cell carcinoma from adenocarcinoma. However, this technique still has obvious limitations, mainly including color overlap of chromogens during colocalization, which forms dark signals that are difficult to interpret, limited antibody species sources (requiring primary antibodies from different species to avoid cross-reaction), and relatively high detection costs. These factors limit its application in complex pathological assessments to a certain extent.
Immunofluorescence
With its wide dynamic range of 5 to 6 orders of magnitude, immunofluorescence (IF) technology can more truly reflect protein expression levels. Theoretically, it is more suitable for precise quantification (such as HER2 low expression detection) and patient stratification for targeted therapy than chromogenic IHC. Currently, it is mainly used in clinical practice for the detection of immunoreactants in autoimmune diseases. However, its popularity in routine pathology laboratories is still limited by many factors, including traditional operation in darkroom environments, low instrument standardization, lack of morphological counterstaining background, autofluorescence interference in FFPE tissues, and easy quenching of fluorescence signals making long-term preservation difficult. Although slide digitization technology has alleviated the problems of operation and archiving to a certain extent, antibody species restrictions and adaptability to FFPE samples still need further optimization.
3. Multiplex Immunohistochemical Staining
Multiplex detection, as a technology capable of simultaneously evaluating two or more markers on the same tissue section, especially multiplex immunofluorescence, has shown potential superior to traditional detection methods in tumor microenvironment research and immunotherapy response prediction. However, its translation to clinical practice still faces many technical bottlenecks, including time-consuming experimental protocol optimization and validation, limited antibody sources from the same species, spatial hindrance effects caused by antibody competition, spectral overlap limiting the number of detectable targets, and the influence of staining sequence and antigen retrieval on results. In addition, complex multiplex data often relies on professional digital image analysis for effective interpretation. These factors together restrict its wide application in routine pathological diagnosis.
Multiplex Immunofluorescence Using Tyramide Signal Amplification
The core principle of Tyramide Signal Amplification (TSA) technology shares commonality with traditional IHC, both relying on enzyme-conjugated secondary antibodies to mediate signal transduction, but innovatively introduces tyramide-conjugated chromogens or fluorophores. Its core advantage comes from the signal amplification effect of enzymatic reactions: when HRP-conjugated enzyme reacts with tyramide, it generates reactive free radicals that drive chromogens or fluorophores to stably bind to proteins near antibody binding sites through covalent bonds. This covalent binding property allows subsequent removal of unbound antibodies through elution steps, creating conditions for multiple rounds of antibody incubation and TSA signal labeling on the same tissue section. Based on this property, TSA technology not only significantly improves detection sensitivity, especially suitable for detection of low-abundance markers, but also enables simultaneous detection of multiple targets by combining fluorophores or chromogens with different spectra. This technology is similar to conventional IHC protocols, can be stained manually or automatically, and most clinical laboratories have basic technical facilities. It is compatible with fresh/frozen and FFPE tissues, can analyze large tissue sections, and fluorescent signals on glass slides can be directly observed for quality control or interpretation, with multiple image analysis devices supporting quantification. However, attention should be paid to protocol optimization in the experimental area, such as staining order, TSA reagent dosage affecting antigenicity and spatial hindrance, and stripping protocols.
Immunohistochemical Cycle Staining
Another important multiplex staining strategy is chromogenic IHC cycle staining technology, among which the more widely used is Multiplex IHC on Single Slide (MICSSS). Its technical core is to break through the target limit of a single round of staining through repeated cycles of "staining-scanning-chemical decolorization". In each cycle, the tissue section is first stained with a specific marker using chromogenic IHC, then digitally scanned and archived, followed by chemical removal of the chromogenic substrate and bound antibodies from the section, and then entering the next cycle of staining-scanning-decolorization for new markers. Finally, through digital image registration and fusion technology, the scanning results of multiple cycles are integrated into a single multi-color digital image. Theoretically, it can detect 10 or more markers in a single experiment, greatly improving the information mining efficiency of a single tissue section, especially suitable for research scenarios that require systematic analysis of co-expression patterns of multiple immune cell subsets or signaling pathway molecules. However, this technology faces significant challenges in clinical application. On the one hand, the time-consuming cycle process significantly limits detection throughput, making it difficult to meet the efficiency requirements of routine clinical diagnosis. On the other hand, multiple cycles of coverslip removal, chemical decolorization, and repeated antigen retrieval steps are likely to lead to degradation of tissue antigenicity, destruction of morphological structure, or even tissue displacement, which not only reduces the image quality of a single round of staining but also affects the accuracy of multiple rounds of image registration. At the same time, this technology has extremely high requirements for the solubility of chromogens, only specific soluble chromogens can be used, limiting the selection range of marker detection. Additionally, the final result is only a digital multi-color scan, not a physical slide that can be archived long-term and reviewed repeatedly. Furthermore, the expression intensity of markers can only be semi-quantitatively evaluated, making it difficult to meet the demand for quantitative data in precision medicine. These factors together restrict its wide application in routine clinical diagnosis.
Virtual Multiplex Technology
As an alternative solution that does not require complex experimental operations, virtual multiplexing digitizes whole-slide images of single chromogenic IHC staining from serial sections of samples, generates multi-color digital sections through software registration and fusion for visual assessment or digital analysis. This technology is based on existing laboratory staining protocols, avoiding the technical challenges of real multiplex staining. Pathologists can locate regions of interest on H&E scanned sections and quickly switch to view multiplex IHC staining results of the same region. However, this technology is still essentially limited by the physical characteristics of serial sections. Due to inevitable cell loss and displacement between sections, it is difficult to precisely match the spatial correspondence of different targets, making true colocalization analysis impossible. Additionally, when a large number of sections are needed, it faces the risk of tissue exhaustion, resulting in obvious limitations in complex pathological assessments relying on precise spatial information.
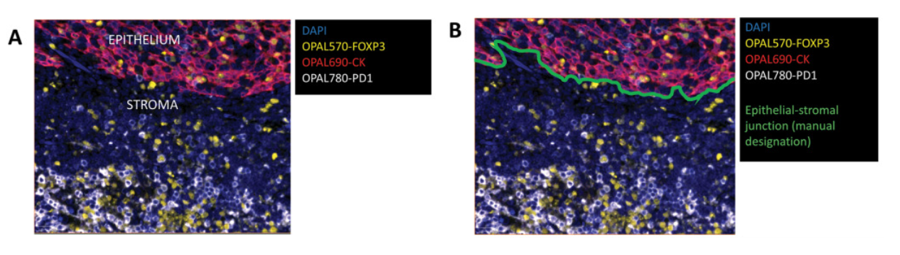
Analysis and Interpretation of Multiplex Images
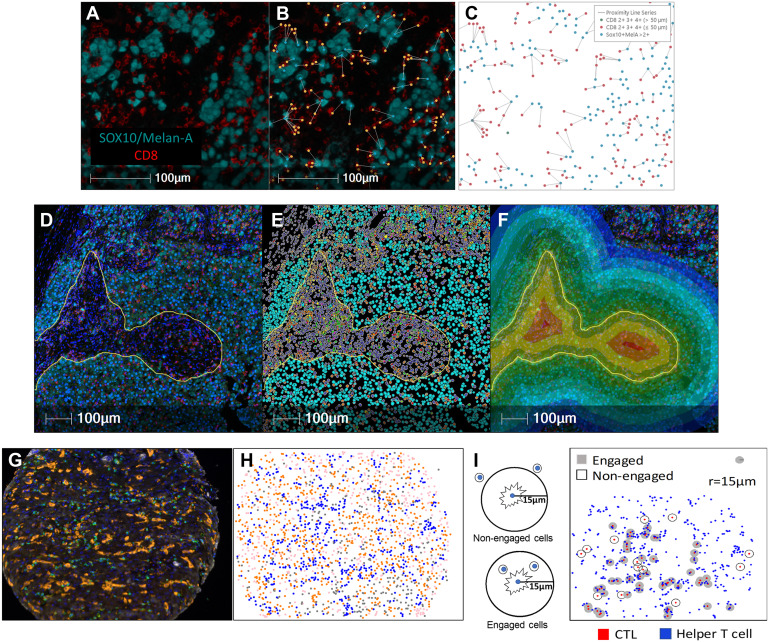
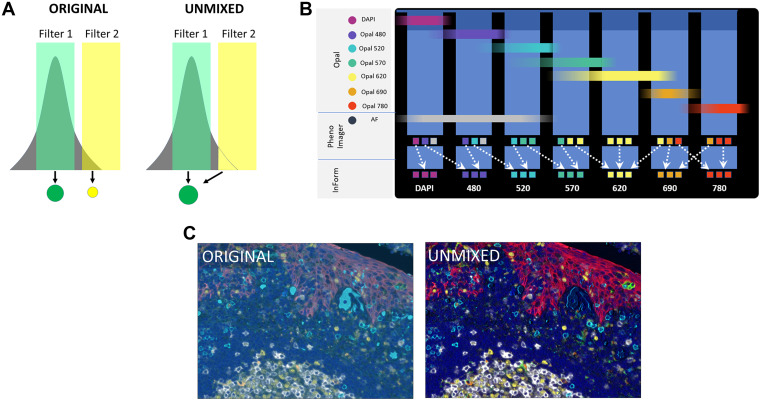
The interpretation methods of multiplex images are divided into direct visual interpretation and digital analysis. Direct visual interpretation is suitable for simple tasks such as tumor immune phenotype analysis. Low-plex chromogenic IHC can be interpreted through glass slides, while multiplex IF requires digital slice interpretation and must rely on digital image analysis technology. This process usually begins with spectral unmixing to eliminate fluorescence crosstalk and focuses the analysis scope by defining regions of interest. Subsequently, data extraction is mainly performed through two core algorithms: one is morphology-based cell segmentation, which uses nuclear dyes to identify individual cells and combines membrane or cytoplasmic signals for phenotypic classification, suitable for cell counting and neighborhood analysis. The other is compartmentalization analysis based on molecular characteristics, which evaluates protein expression in specific subcellular structures or extracellular matrix through pixel intensity, independent of complete cell morphology. However, digital analysis still faces many challenges, including non-specific background signal interference, errors in cell segmentation algorithms when dealing with irregular cells or non-sectioned nuclei, and limitations of pixel-based analysis in distinguishing individual cells. These factors may affect the accuracy of final quantitative results.
4. Summary
Although multiplex detection technology is still in its infancy in clinical applications, its enormous potential in tumor diagnosis classification and immunotherapy prognosis assessment is driving the development of this field. Particularly in prognostic analysis, multiplex combinations centered on PD-1/PD-L1 and immune cell markers have been clinically validated and are expected to become companion diagnostic tools for guiding immunotherapy. In terms of technical approaches, bright-field multiplex IHC based on TSA is expected to be the first to be popularized in clinical laboratories due to its compatibility with existing pathology workflows. However, given the maturity and cost advantages of single and dual detection technologies, multiplex detection will serve as an important supplement rather than a complete replacement for traditional methods in the short term.
References
Harms PW, Frankel TL, Moutafi M, Rao A, Rimm DL, Taube JM, Thomas D, Chan MP, Pantanowitz L. Multiplex Immunohistochemistry and Immunofluorescence: A Practical Update for Pathologists. Mod Pathol. 2023 Jul;36(7):100197. doi: 10.1016/j.modpat.2023.100197. Epub 2023 Apr 25. PMID: 37105494.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
