Why Use Secondary Antibodies? — Selection and Application of Secondary Antibodies
Secondary antibodies, are a class of antibodies that can specifically recognize the constant region of the primary antibody. In the immunoassay system, the core task of primary antibodies is to directly bind to the target antigen, which is equivalent to a "detective who accurately finds the target". The secondary antibody does not act directly with the antigen, but specifically binds to the primary antibody, and converts the binding signal of the primary antibody to the antigen into a detectable signal through the markers it carries.
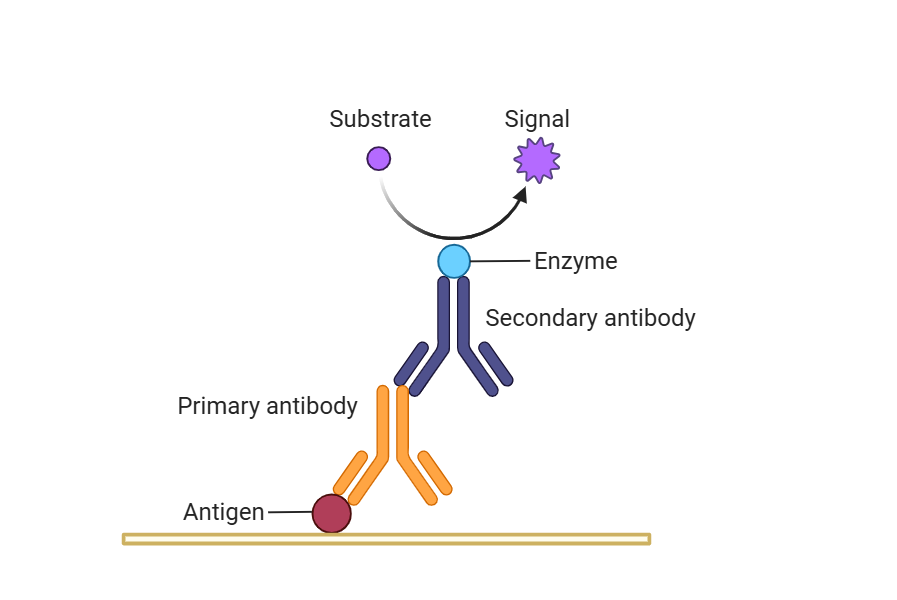
The necessity of using secondary antibodies
In most immunoassay experiments, there are often many limitations to using primary antibodies for detection. For example, traditional monoclonal primary antibodies are usually unlabeled and cannot directly capture their binding signal to antigens through instruments; Labeling each primary antibody separately is not only costly and cumbersome, but may also affect the binding activity of the primary antibody. The emergence of secondary antibodies perfectly solves these problems, and its core role can be summarized in four points:
First, signal amplification. This is one of the core values of secondary antibodies. An antigen molecule can bind to multiple primary antibodies at the same time, and a primary antibody molecule can be recognized and combined by multiple secondary antibodies, forming a multi-stage amplification system of "antigen - primary antibody - secondary antibody", which can significantly enhance the weak antigen signal, especially suitable for the detection of low-abundance antigens, and greatly improve the sensitivity of experiments.
Second, simplify experimental design. There is no need to label each specific primary antibody, but only need to prepare a labeled secondary antibody for a certain type of primary antibody (such as mouse-derived and rabbit-derived), which can be adapted to the detection of a variety of target antigens, which greatly reduces the experimental cost and shortens the experimental cycle.
Third, improve universality. The same labeled secondary antibody can be matched with multiple primary antibodies from the same source, such as rabbit secondary antibodies that can bind to all rabbit-derived primary antibodies, regardless of the antigen they target. This versatility eliminates the need for separate labeling reagents for different antigen tests, significantly improving experimental efficiency.
Fourth, expand the detection method. Different markers carried by secondary antibodies can be adapted to a variety of detection platforms. Fluorescently labeled secondary antibodies are suitable for immunofluorescence microscopy observation and flow cytometry analysis. Enzyme-labeled secondary antibodies can be applied to ELISA, Western Blot, immunohistochemistry (IHC) and other experiments through chromogenic reactions. Biotin-labeled secondary antibodies can be combined with avidin-marker systems to further amplify the signal and meet the needs of higher sensitivity detection.
Scientifically select secondary antibodies
Selecting the appropriate secondary antibody is the core link to ensure the accuracy of experimental results, focusing on the characteristics of the primary antibody, experimental methods and detection needs, mainly considering the following factors:
1. Match the source species of the primary antibody
The primary principle for selecting a secondary antibody is to "target the host species of the primary antibody". For example, if the primary antibody used is a rabbit-derived antibody, an "anti-rabbit secondary antibody" should be chosen; if the primary antibody is mouse-derived, the corresponding "anti-mouse secondary antibody" should be selected. This matching relationship directly determines whether the secondary antibody can specifically recognize the primary antibody, preventing issues such as the absence of binding signals or non-specific binding.
2. Match the antibody subtype of the primary antibody
The antibody subtypes of primary antibodies mainly include IgG, IgM, IgA, etc., among which IgG is the most commonly used type in immunoassays, so anti-IgG secondary antibodies are preferred in most cases. If an IgM-type primary antibody is used in the experiment, it is necessary to select the anti-IgM secondary antibody specifically to ensure binding specificity. In addition, some secondary antibodies can also recognize subclasses of IgG (e.g., IgG1, IgG2a, IgG2b), which are suitable for experiments with special requirements for antibody subclasses.
3. Select markers according to the experimental method
Fluorescent labels, such as FITC, Cy3, and the Alexa Fluor series, have high stability and strong specificity of fluorescent signals, and are suitable for experiments that require direct observation of signal localization or quantitative analysis, such as immunofluorescent staining and flow cytometry.
Enzyme labels, such as HRP and AP, realize signal detection by catalyzing substrates to produce chromogenic or luminescent reactions, and are applicable to experiments like ELISA, Western Blot, and immunohistochemistry. Among them, HRP is the most widely used due to its high catalytic efficiency and low cost.
Biotin labels do not directly generate detectable signals themselves, but can specifically bind to avidin or streptavidin. Then, by combining with enzyme- or fluorescent-labeled avidin, secondary amplification of signals can be achieved, making them suitable for high-sensitivity detection of low-abundance antigens.
4. Pay attention to specificity and purification methods
The specificity of the secondary antibody directly affects the level of the experimental background signal, and the affinity purification level of the secondary antibody is preferred. Affinity purification refers to the purification of serum containing antibodies through a porous purification resin column with a solid-phase ligand. Affinity-purified antibodies are generally preferred because these products are more specific and produce less non-specific bands and backgrounds.
5. Avoid species cross-reactivity
If the experimental sample contains protein components of other species, the cross-reactivity characteristics of the secondary antibody should be confirmed in advance. For example, if a murine primary antibody is used to test human samples, the selected anti-murine secondary antibody should be clearly marked as "does not cross-react with human IgG" to avoid binding the secondary antibody to human IgG in the sample and interfering with the experimental results.
Guide to Avoiding Pitfalls in the Use of Secondary Antibodies: Key Precautions
1. Strictly control the dilution ratio
The dilution ratio of secondary antibodies should strictly follow the product instructions, and it is recommended to explore the optimal concentration through pre-experiments. Too high a dilution ratio can lead to insufficient concentration of the secondary antibody, and the signal is weak or even undetectable. Too low a dilution ratio will increase non-specific binding, resulting in an increase in background signal and affecting the judgment of results.
2. Optimize incubation conditions
Most secondary antibodies are incubated at room temperature for 1-2 hours, and some experiments can be incubated overnight at 4°C to enhance binding specificity. It is necessary to avoid incubation at high temperatures (exceeding 37°C) or incubation for too long, otherwise it is easy to lead to an increase in non-specific binding of secondary antibodies and interfere with the experimental results.
3. Pay attention to the washing steps
After incubation, the reaction system should be thoroughly washed with washing solution, typically 3-5 times. Adequate washing can remove unbound free secondary antibodies, which is a key step to reduce background interference and ensure experimental accuracy, and cannot be simplified or omitted.
4. Standardize preservation conditions
The activity of secondary antibodies is easily affected by temperature, repeated freezing and thawing, and it is recommended to store them according to the requirements of the instructions. Unopened secondary antibodies usually need to be frozen at -20°C, and can be divided into small volumes after opening to avoid repeated freezing and thawing, and can be refrigerated at 4°C for short-term use. At the same time, attention should be paid to avoiding strong light exposure, especially for fluorescently labeled secondary antibodies, which need to be avoided throughout the whole process, and avoid strong light exposure during incubation and washing. Minimize exposure time during inspection.
5. Set up control experiments
In order to verify the specificity of the secondary antibody, a control group without a primary antibody should be set up in the experiment. If there is a clear signal in the control group, it indicates that there is non-specific binding of the secondary antibody, and the experimental conditions need to be re-optimized or the secondary antibody needs to be replaced.
6. Avoid reuse
Used secondary antibodies may contain unbound impurities and degrading components, and the concentration is difficult to control accurately, which will lead to signal instability and background elevation, affecting the reliability of experimental results.
Our company offers more than 400 secondary antibodies, including multiple sources and multiple subtypes. In addition, we offer 30 labeling kits that can be used to label secondary antibodies to meet your experimental needs. Visit our website to find the perfect secondary antibody for your target.
 | Felicia Felicia is a technical support specialist at EnkiLife, with extensive professional experience in antibody development, optimization, and ELISA assay design and application. She is committed to assisting our clients in selecting suitable antibody products, optimizing ELISA experimental protocols, and resolving technical challenges encountered in the process, thereby supporting the smooth progress of their life science research projects. |
