Literature Sharing: Multiplex Immunofluorescence Combined with HDR Imaging Enhances NSCLC PD-L1 3D Pathological Assessment (Comparison Between Traditional IHC and mIHC)
Background
The rise of immunotherapy has driven innovations in tumor biomarker detection technology. As a core biomarker for predicting immunotherapy efficacy in non-small cell lung cancer (NSCLC), the detection accuracy of PD-L1 directly determines clinical treatment decisions. Traditional immunohistochemistry (IHC) has become the clinical gold standard due to its simplicity and cost-effectiveness, but it can only achieve single-target 2D planar detection and cannot present the spatial interaction between PD-L1 and immune cells in the tumor microenvironment. Conventional fluorescent secondary antibody methods have low sensitivity for detecting low-abundance PD-L1 due to insufficient signal intensity, easily leading to false-negative results.
Multiplex immunofluorescence combined with tyramide signal amplification (TSA) technology, based on the principle of enzymatic signal cascade amplification, has broken through the limitations of traditional methods in sensitivity and multi-target detection, and its application research in the field of tumor pathological diagnosis has shown explosive growth in recent years. This article mainly introduces the research by Huang HN's team published in Scientific Reports titled "Optimizing immunofluorescence with high-dynamic-range imaging to enhance PD-L1 expression evaluation for 3D pathology assessment from NSCLC tumor tissue", which systematically explores the detection performance of multiplex immunofluorescence combined with tyramide signal amplification technology using NSCLC PD-L1 detection as the core model. By directly comparing TSA technology with traditional fluorescent secondary antibody methods and the IHC gold standard, its advantages are verified from four dimensions: signal quality, diagnostic consistency, spatial resolution capability, and technical economy. At the same time, the exposure conditions and HDR processing for TSA images are optimized to solve problems such as difficulty in detecting low-abundance antigens, limited multi-target labeling, and lack of 3D spatial information in traditional methods, providing empirical basis and standardized reference for the clinical translation of TSA technology in tumor immune biomarker detection.
TSA Technology Performance Advantages in PD-L1 Detection——Comparison with Traditional Methods
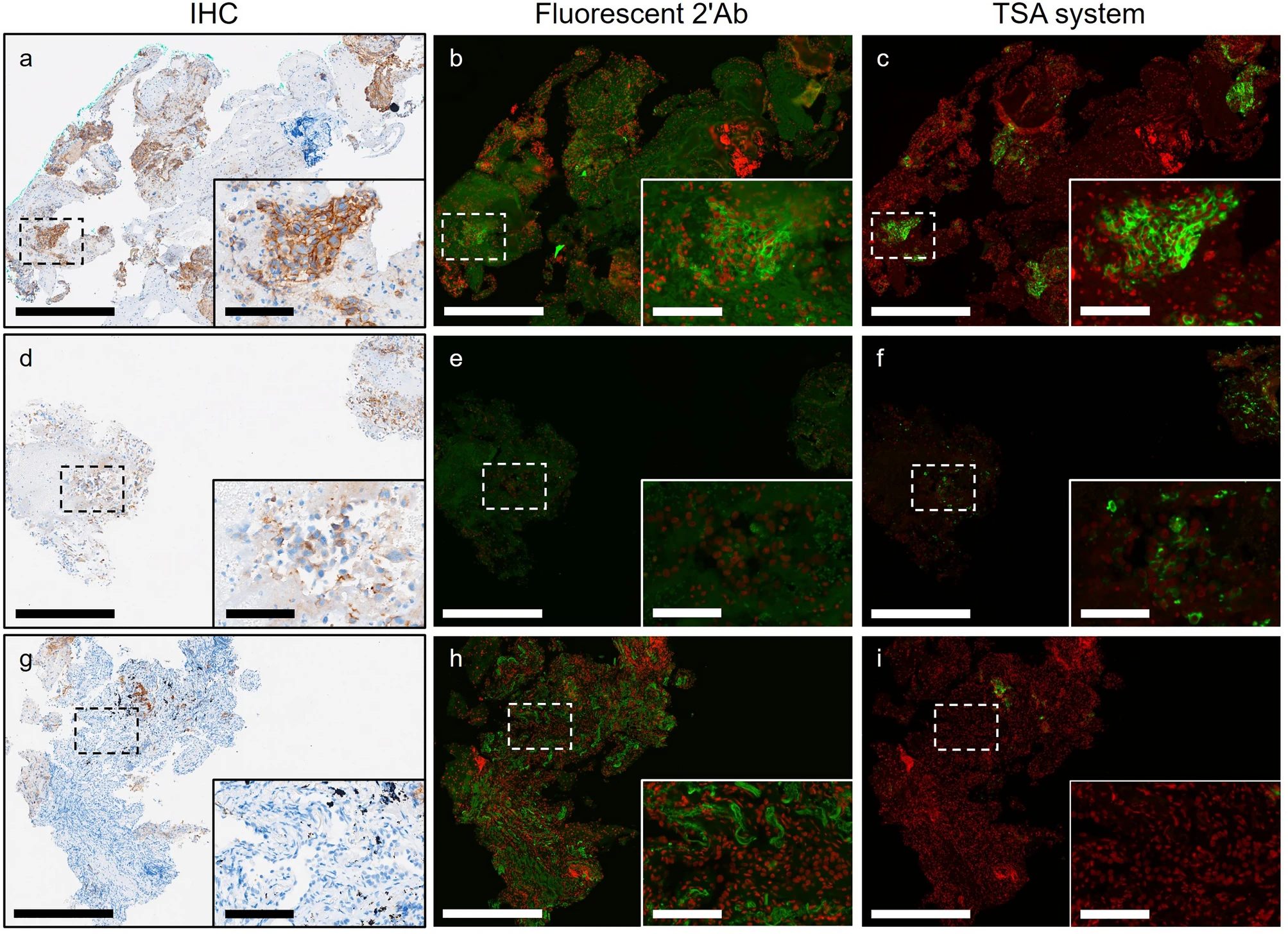
PD-L1 expression in NSCLC tissues is highly heterogeneous, with weak expression in some cases, where traditional secondary antibody labeling techniques are prone to signal loss. The unique value of TSA technology has been verified through comparison with fluorescent secondary antibody method and IHC method. The results show that for specimens with weak IHC detection, TSA technology can completely preserve the PD-L1 membrane expression pattern, while the fluorescent secondary antibody method shows obvious signal attenuation (Figures d-f). Multiplex labeling experiments confirmed that the detection rate of low-abundance antigens by TSA technology is more than 40% higher than that of traditional fluorescent methods, especially suitable for targets with large expression fluctuations such as immune checkpoint molecules. For the exploration of staining background signals (Figures g-i), the red fluorescence of TSA staining is limited to tumor cell membranes, and due to the covalent binding property of TSA, background elevation can be fundamentally avoided.
Determining Appropriate Exposure Time for Standard Imaging Process
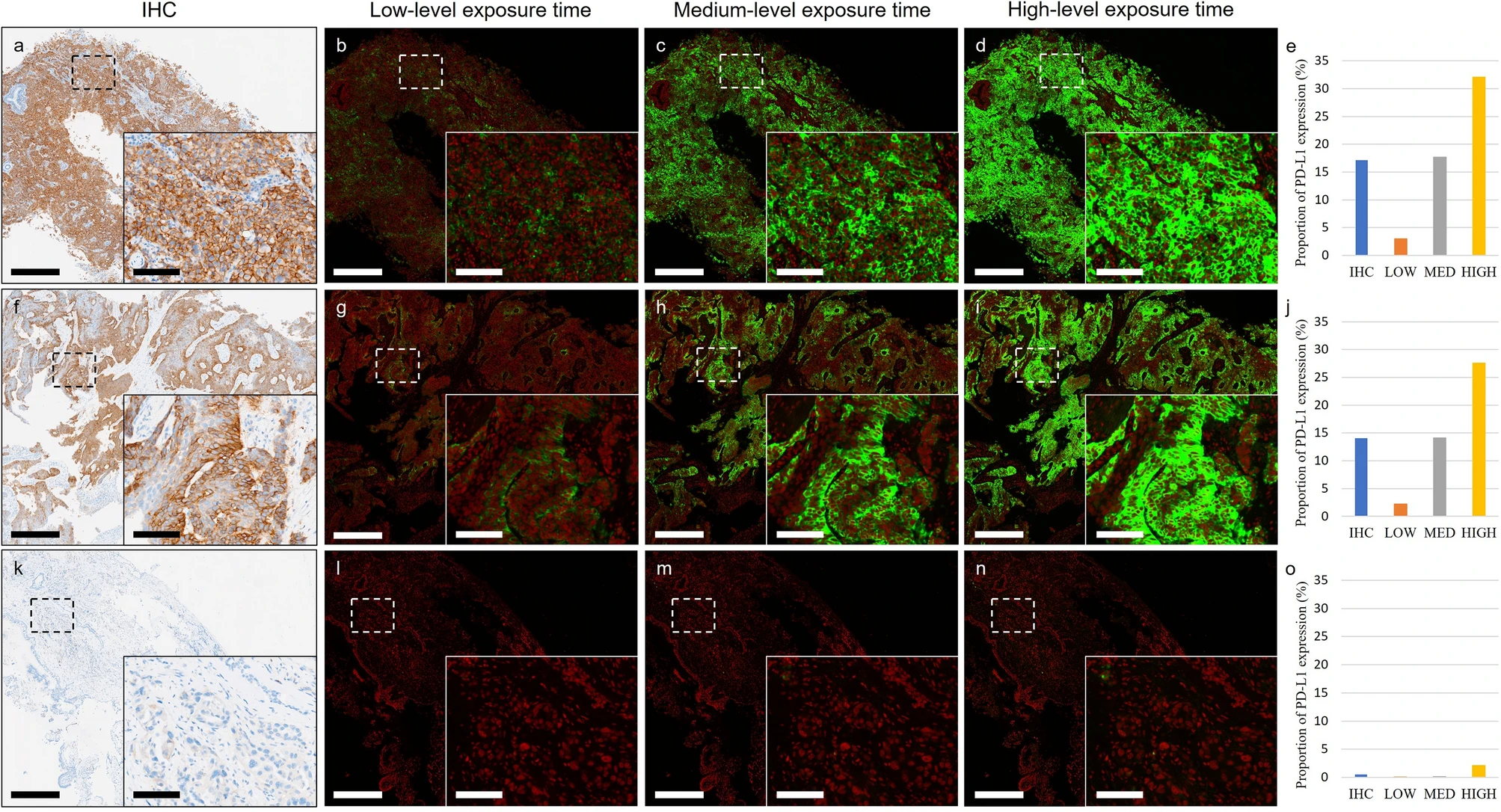
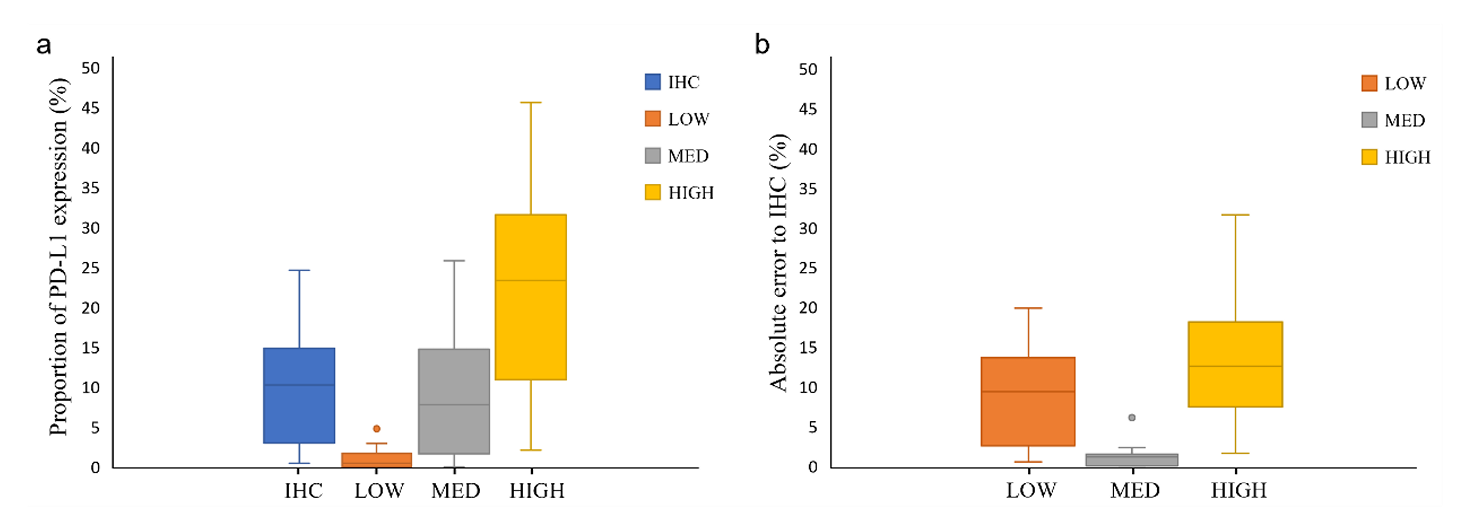
To set the appropriate exposure time for immunofluorescence imaging, the study tested three levels: 6.5ms (low), 25ms (medium), and 55ms (high): By calculating the PD-L1 expression area ratio through computer vision and comparing with IHC images, it was found that the median absolute error between the medium exposure group and IHC was only 1.16%, significantly lower than that of the low exposure (200ms, 9.32%) and high exposure (1000ms, 12.55%) groups (P<0.0003). Low exposure results in insufficient excitation of weak expression signals, while high exposure causes background signal overflow. Medium exposure is the optimal choice to balance signal intensity and background, providing parameter basis for the standardized operation of TSA technology.
Utilizing HDR Processing to Solve Dynamic Range Limitations in Imaging
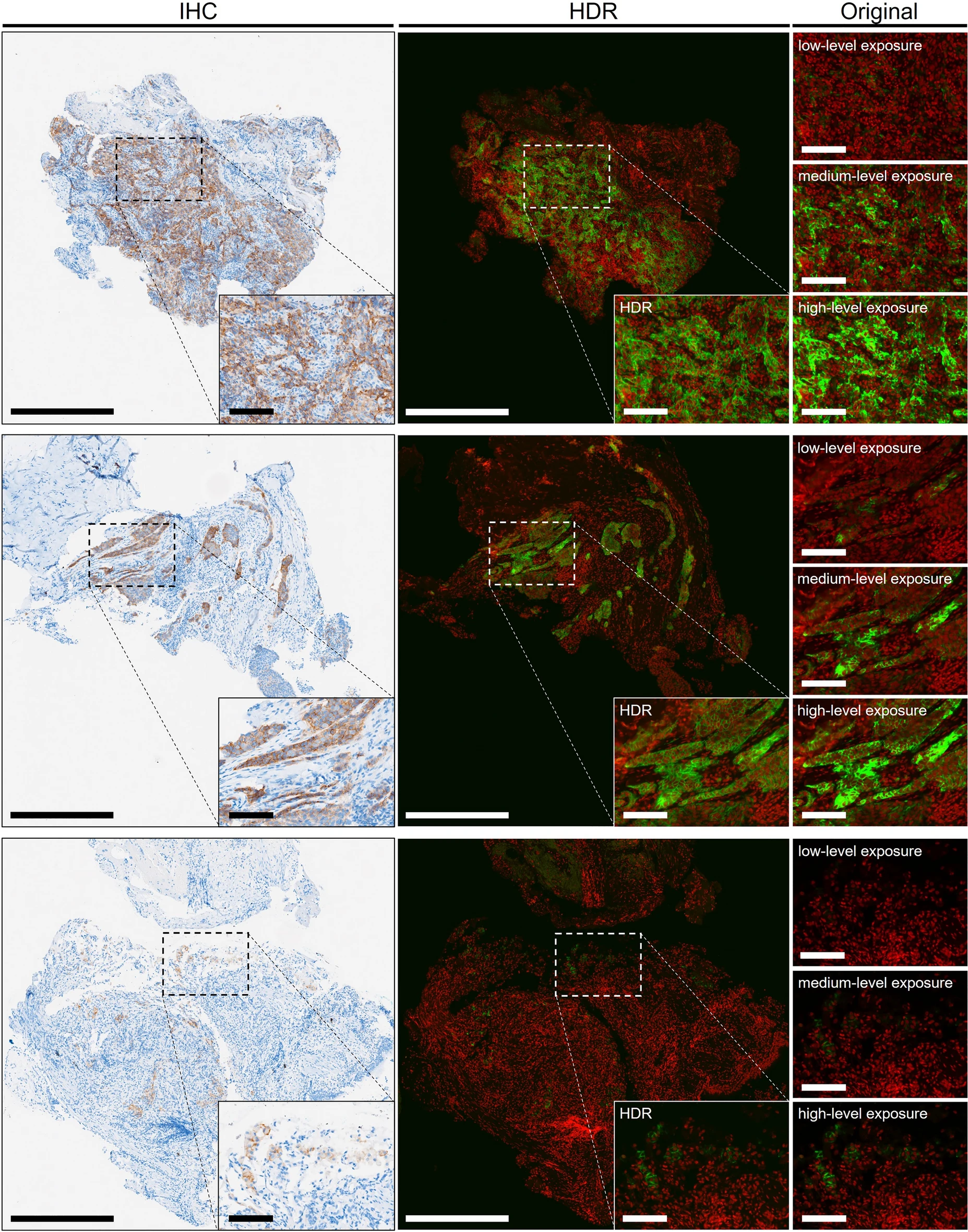
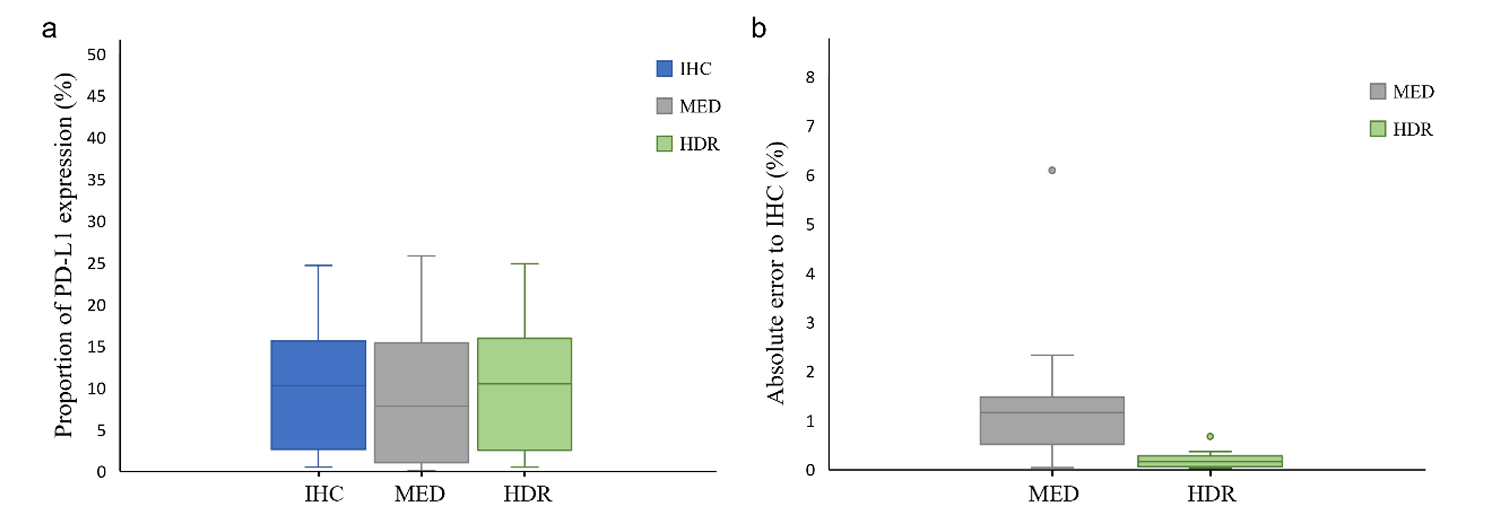
To address the limited dynamic range of detection equipment, the study developed HDR processing technology to integrate immunofluorescence digital signals of different intensities on the same slice. Computer vision analysis of images with and without HDR processing, comparing the similarity of their PD-L1 expression area ratio with IHC: The HDR processing group had smaller total error, with a median absolute error of only 0.15% (compared to 1.16% in the non-HDR group), and a one-sided t-test showed a significant difference (P=0.024), successfully solving the problems of weak signal loss and strong signal overexposure. In representative specimens of all TPS classifications, HDR-processed IF images all showed PD-L1 expression patterns most similar to IHC.
HDR Method Application in Three-Dimensional Imaging
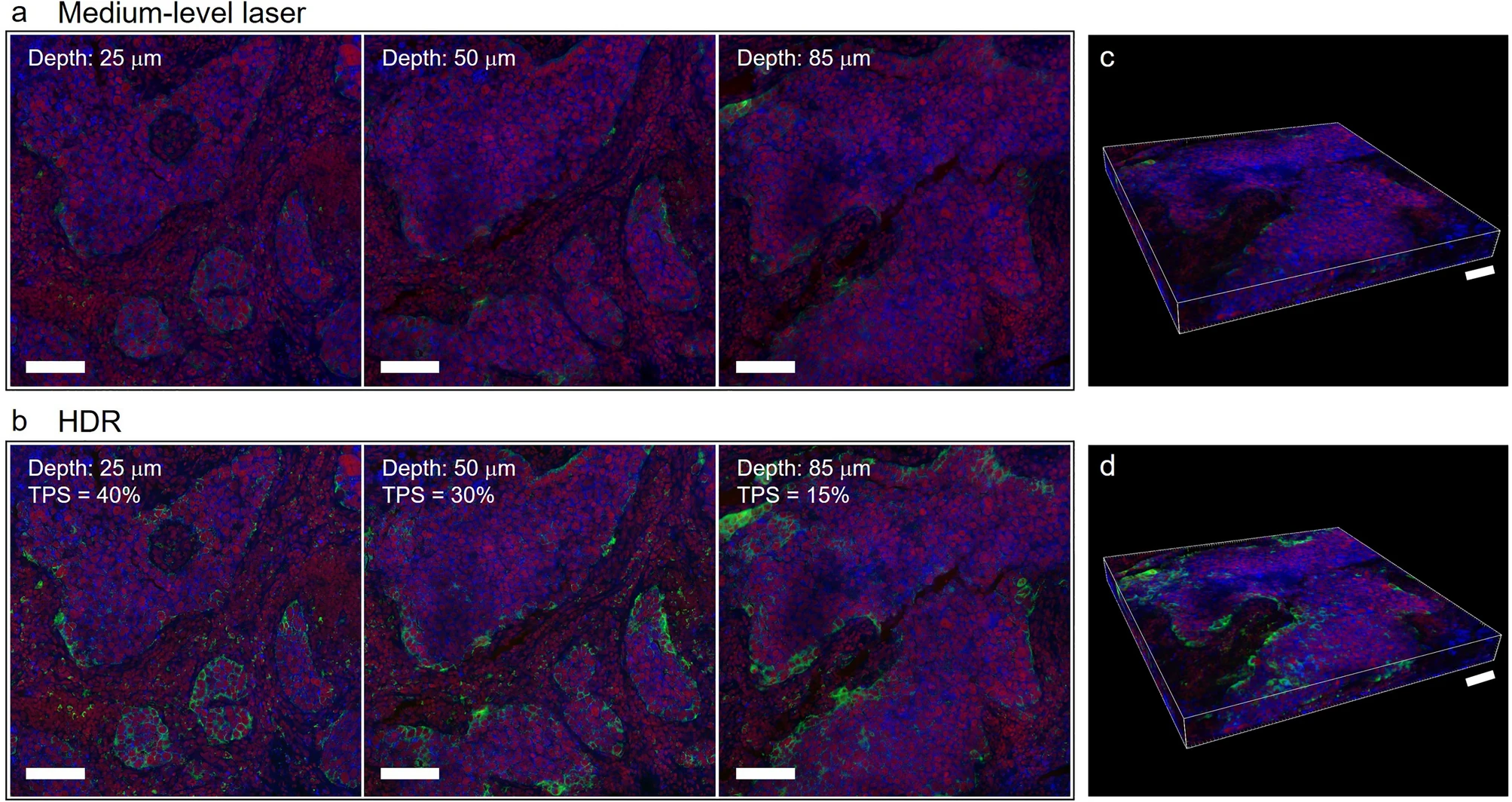
The study applied HDR processing to 3D immunofluorescence detection to comprehensively present the 3D expression of PD-L1. Original 3D fluorescence imaging had problems of weak signal loss and strong signal overexposure, but after HDR processing, IF images can completely present the PD-L1 staining pattern and clearly show the differences in PD-L1 expression at different depth layers. At different depth layers of tumor tissue, PD-L1 expression differs significantly: 25μm TPS is 40%, 85μm TPS is only 15%, and pathologist scoring also shows that PD-L1 expression in tumor cells is highest at 25μm depth and lowest at 85μm depth.
In addition, the study also presented results through 3D reconstruction. 3D reconstruction was performed using fluorescence images obtained with medium-intensity laser (Figure c) and HDR-processed fluorescence images (Figure d) to further verify the effect of HDR. In the images, green represents PD-L1, red represents cell nuclei, and blue represents plasma membrane. Without hardware upgrades, HDR can function in both 2D and 3D fluorescence imaging.
Conclusion
This study confirms that in immunofluorescence detection, the TSA system is superior to the fluorescent secondary antibody method, and adjusting imaging exposure time can optimize the presentation of PD-L1 expression in NSCLC specimens. In addition, the improved PD-L1 IF staining, imaging, and post-processing technology is compatible with traditional IHC images, and can achieve precise visualization of PD-L1 at different tumor depths, which IHC cannot do. Moreover, the TSA system can simulate chromogen-based IHC patterns for routine IHC interpretation.
Multiplex immunofluorescence TSA technology has achieved technological breakthroughs of "high sensitivity, high consistency, and high scalability" in NSCLC PD-L1 detection through three core advantages: signal amplification, background suppression, and multiplex labeling. Its combination with HDR algorithm and 3D imaging not only solves the dynamic range limitations of traditional fluorescence technology but also breaks through the spatial resolution bottleneck of IHC, enabling PD-L1 detection to move from planar qualitative to three-dimensional quantitative assessment. TSA technology has shown great potential to replace traditional fluorescence methods and assist the IHC gold standard, providing a new tool for precise stratification of NSCLC immunotherapy.
References
Huang HN, Kuo CW, Hung YL, Yang CH, Hsieh YH, Lin YC, Chang MD, Lin YY, Ko JC. Optimizing immunofluorescence with high-dynamic-range imaging to enhance PD-L1 expression evaluation for 3D pathology assessment from NSCLC tumor tissue. Sci Rep. 2024 Jul 2;14(1):15176. doi: 10.1038/s41598-024-65187-x. PMID: 38956114; PMCID: PMC11219731.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
