Literature Sharing: Application of Multiplex Immunofluorescence in the Study of Tumor Tertiary Lymphoid Structures
Background
Tertiary Lymphoid Structures (TLSs) are ectopic lymphoid aggregates that can develop within or adjacent to tumors, activating anti-tumor immunity. Their maturity status is closely related to patients' immunotherapeutic efficacy and survival, making them highly promising prognostic markers. However, TLSs research has long been constrained by technical bottlenecks: First, traditional single-marker immunohistochemistry cannot distinguish functional TLSs from ordinary immune aggregates, resulting in high misjudgment rates. Second, the lack of simultaneous multi-dimensional marker evaluation makes it difficult to establish maturity grading standards. Third, the incompatibility between preclinical mouse models and patient sample detection techniques hinders translational research.
Opal-TSA multiplex immunofluorescence (mIF) technology, with its high sensitivity and staining flexibility, has become an ideal choice to break through these bottlenecks. The literature "Protocol for investigating tertiary lymphoid structures in human and murine fixed tissue sections using Opal-TSA multiplex immunohistochemistry" centers on Opal-TSA multiplex immunofluorescence technology, constructing a standardized research protocol for TLSs in human and mouse tumor tissues. As key ectopic lymphoid aggregates in the tumor microenvironment, the precise identification and functional characterization of TLSs are crucial for tumor immunity research. Through the innovative application of mIF technology, this literature provides a reliable path to solve technical bottlenecks in TLSs research.
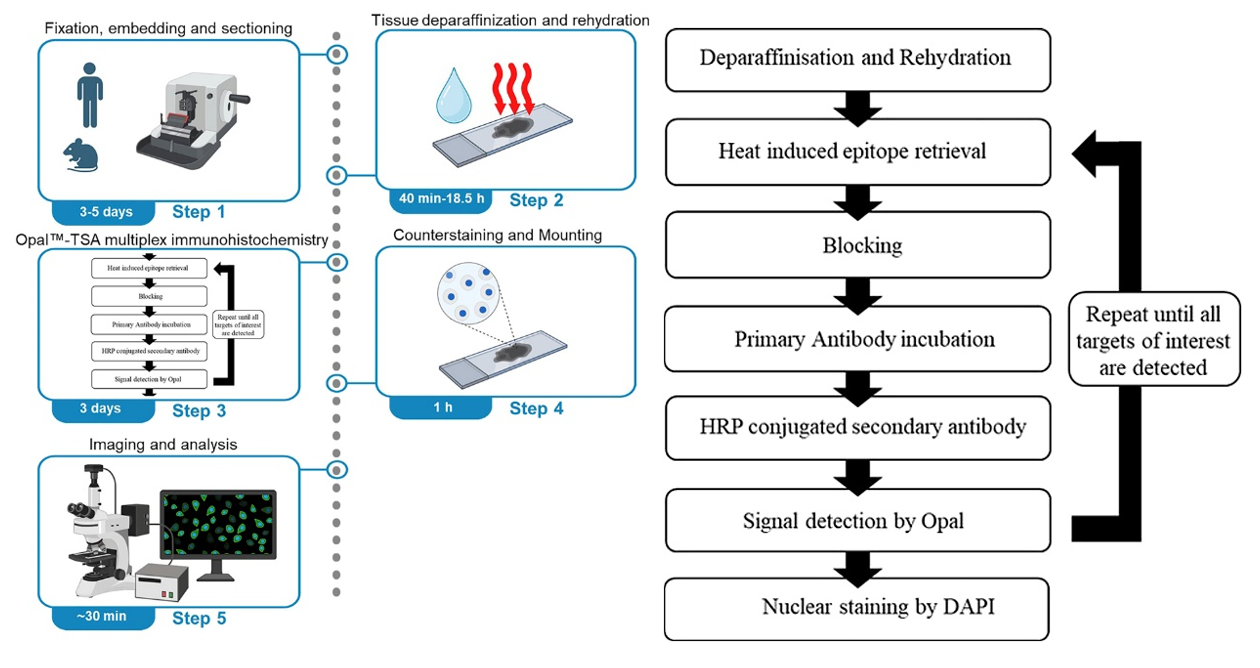
Experimental Design
To determine the maturity of TLSs, the authors selected antibodies CD4, CD8, CD19, CD21, DC-LAMP, PNAd, and DAPI for seven-color labeling of TLSs in human formalin-fixed paraffin-embedded tissue sections. In mouse tissue sections, the DC-LAMP antibody was removed for six-color labeling of TLSs. This covers the core cellular composition of TLSs while distinguishing TLSs from ordinary immune cell aggregates through marker co-localization. The matching of fluorophores follows the principle of low-abundance antigens paired with high-brightness fluorophores: low-abundance markers with lower expression levels are paired with brighter fluorophores such as Opal™620 or Opal™520, while high-abundance markers with higher expression levels are paired with relatively dimmer fluorophores such as Opal™690. The staining order was then determined through heat stress tests. Using optimized primary antibody conditions, 6 consecutive positive control sections were each subjected to 1-6 rounds of Heat-Induced Epitope Retrieval (HIER) treatment before staining. Under fixed exposure time, the differences in staining intensity of the sections were observed: markers sensitive to HIER were detected first, while markers resistant to HIER were detected later. A 50°C low-pH glycine buffer was used for antibody elution to ensure complete removal of the previous round of antibodies without affecting the already bound fluorescent signals. After staining, panoramic scanning and multispectral imaging were performed using the Vectra automated quantitative pathology imaging system, combined with Phenochart, inForm, and HALO software for image spectral unmixing, cell phenotype analysis, and quantitative statistics.
Multi-marker Co-localization
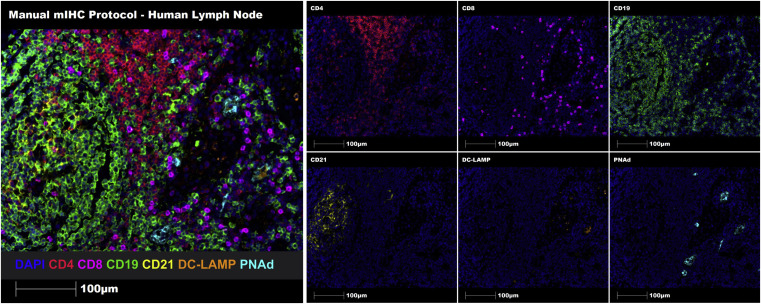
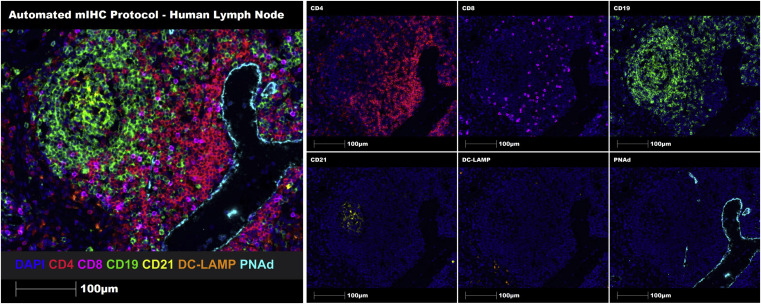
Through seven-marker Opal-TSA technology, multiplex staining was performed on representative human lymph node FFPE tissue sections. Both manual and automated staining successfully identified and characterized immune cell aggregates and TLSs on FFPE tissue sections.
Constructing a Maturity Grading System for Human Tumor TissueTLSs
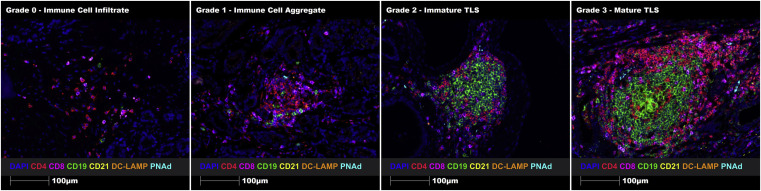
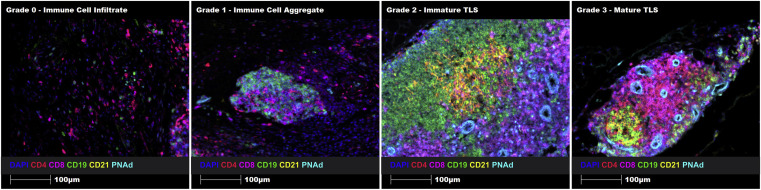
To construct a TLSs maturity grading system, the article designed a comparative diagram of TLSs grading in different regions of the same tissue. Four typical regions were selected from human prostate cancer FFPE tissue sections: immune cell infiltration (Grade 0), immune cell aggregation (Grade 1), immature TLS (Grade 2), and mature TLS (Grade 3). Intuitive quantification of grading standards was achieved through uniform marker staining and imaging parameters.
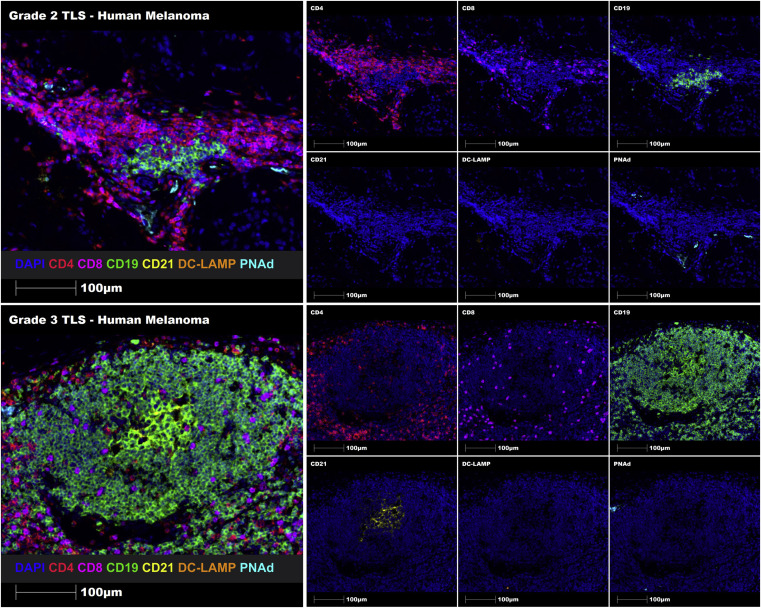
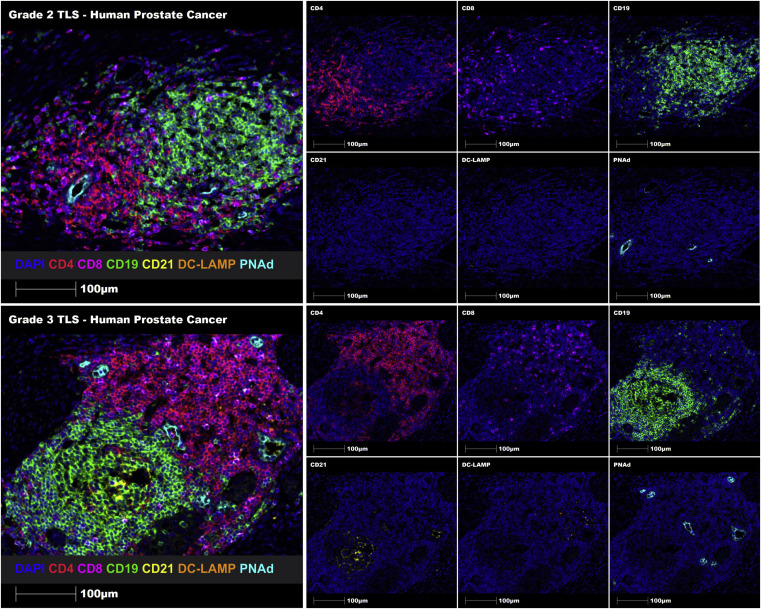
Using the proposed TLSs grading system, Grade 2 and Grade 3 TLSs were successfully identified in melanoma FFPE tissue sections through manual staining. Additionally, automatic staining was successfully implemented in human prostate cancer FFPE tissue sections. This demonstrates that the constructed TLSs maturity grading system can identify and grade immune cell aggregates, immature TLSs, and mature TLSs in human FFPE tumor tissue sections.
Cross-species Image Validation of TLSs Maturity Grading System
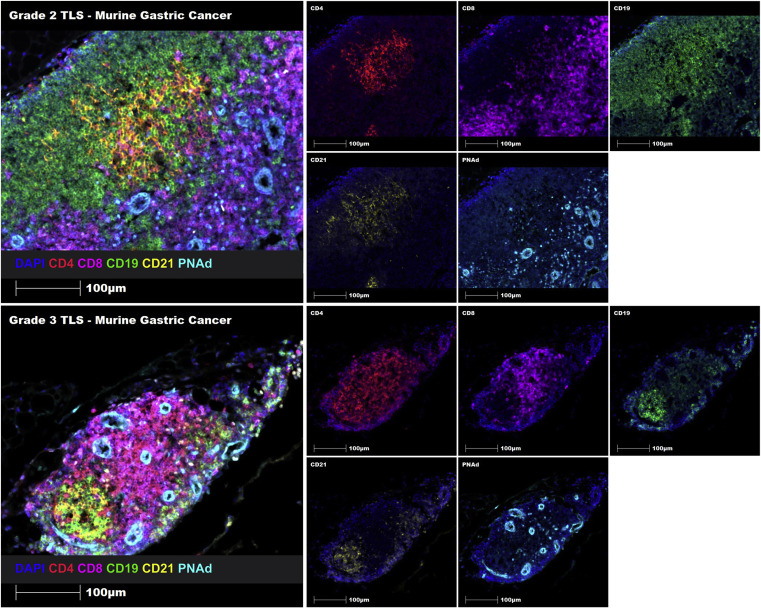
To verify the cross-species adaptability of the protocol, the article simultaneously presents mIF images of mouse gastric cancer tissue sections using six-color labeled Opal-TSA. The image results show accurate identification and classification of TLSs maturity in mouse tissue sections, including immune cell infiltration, immune cell aggregation, immature TLS, and mature TLS. Similarly, Grade 2 and Grade 3 TLS can also be successfully identified in mouse gastric cancer tissue sections through manual staining.
The value of cross-species images lies not only in verifying technical universality but also in building a bridge for correlational studies between "preclinical mouse models - clinical patient samples". This allows research results on TLSs regulatory mechanisms in mouse models to preliminarily predict their application prospects in humans through image feature analogy, accelerating the process of translational basic research to clinical practice.
Summary
Through the innovative application of multiplex immunofluorescence images, the authors upgraded mIF technology from a simple staining method to an image-driven research tool. They constructed an experimental protocol based on Opal-TSA multiplex immunohistochemistry technology that enables in situ detection and characterization of TLSs in human and mouse tissue sections. This protocol supports simultaneous detection of up to 6 antigen markers and provides a supporting grading system for distinguishing immature and mature TLSs, offering a complete technical workflow from detection to result interpretation for TLSs in analyzing the tumor immune microenvironment and evaluating immunotherapeutic efficacy.
References
Quigley LT, Pang L, Tavancheh E, Ernst M, Behren A, Huynh J, Da Gama Duarte J. Protocol for investigating tertiary lymphoid structures in human and murine fixed tissue sections using Opal™-TSA multiplex immunohistochemistry. STAR Protoc. 2023 Mar 17;4(1):101961. doi: 10.1016/j.xpro.2022.101961. Epub 2023 Jan 10. PMID: 36633948; PMCID: PMC9843255.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
