Product Introduction
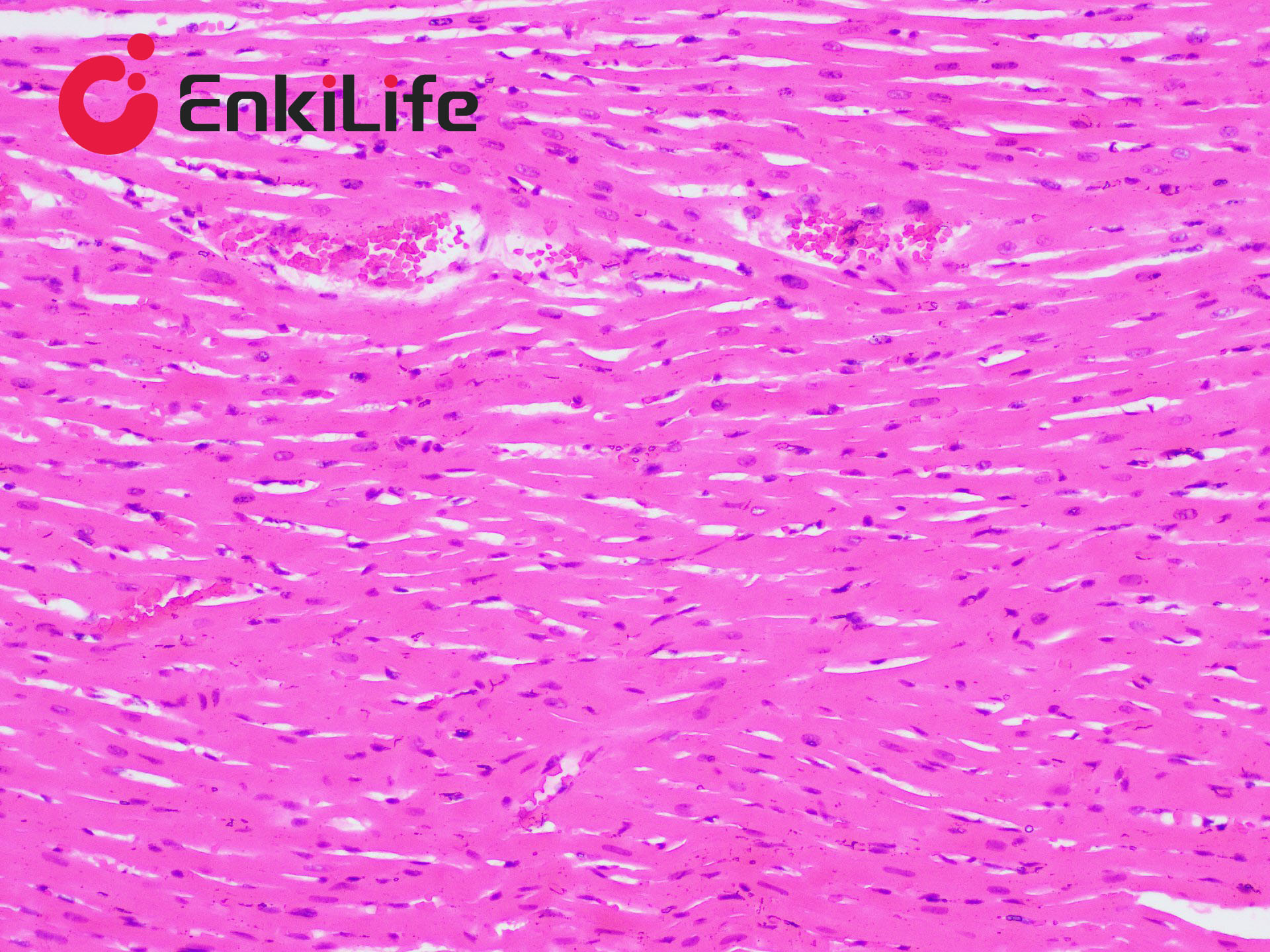
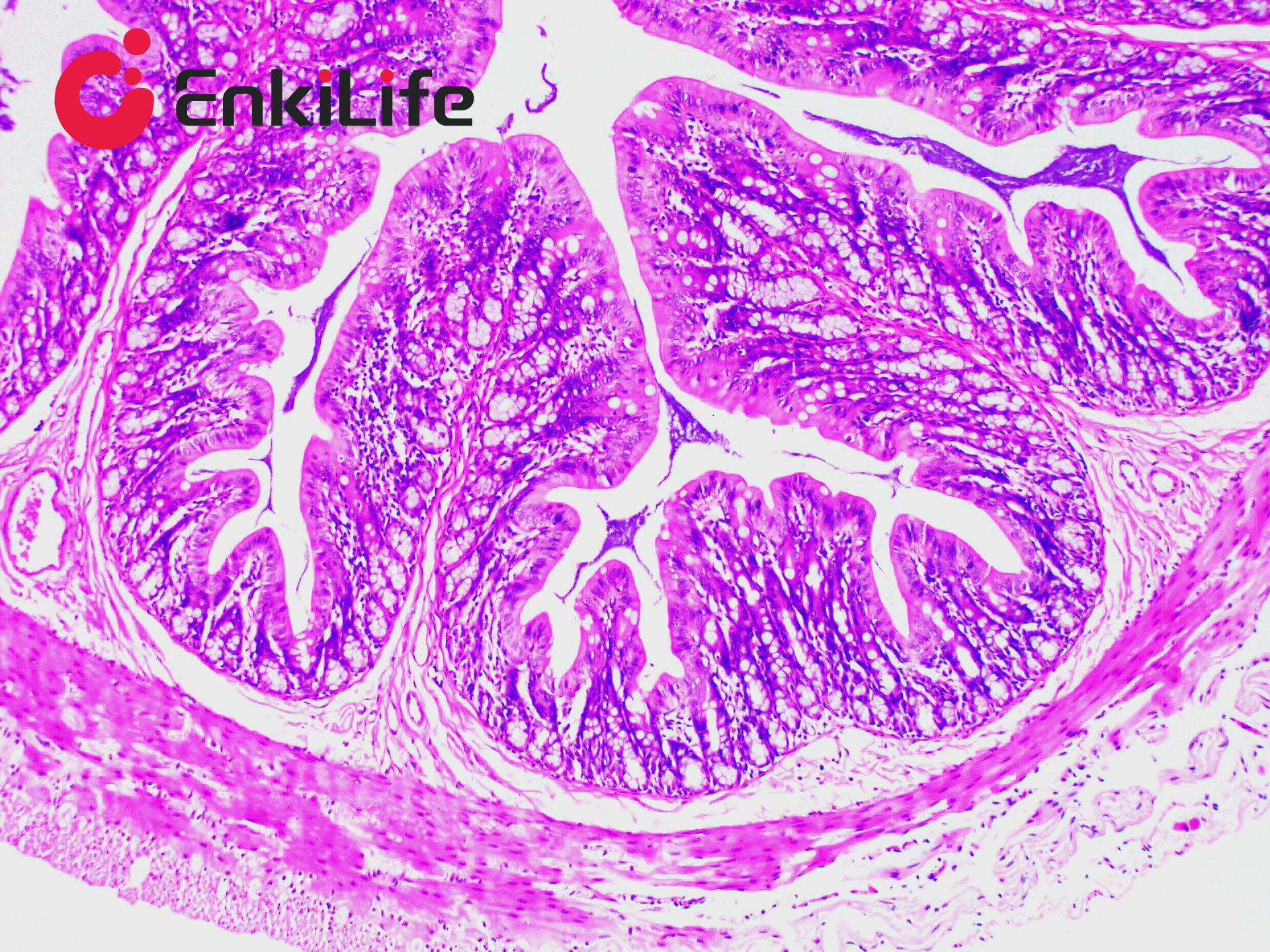
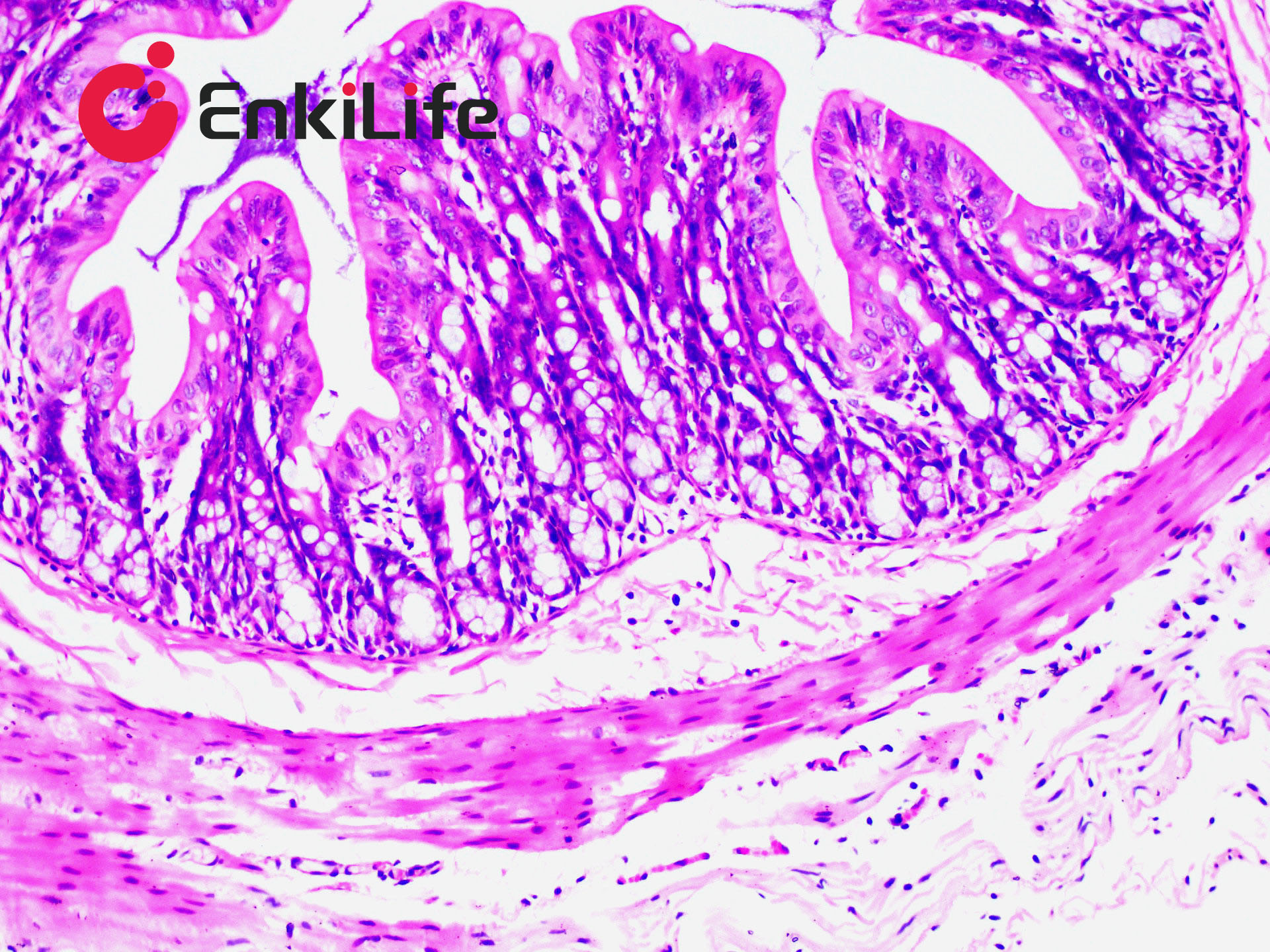
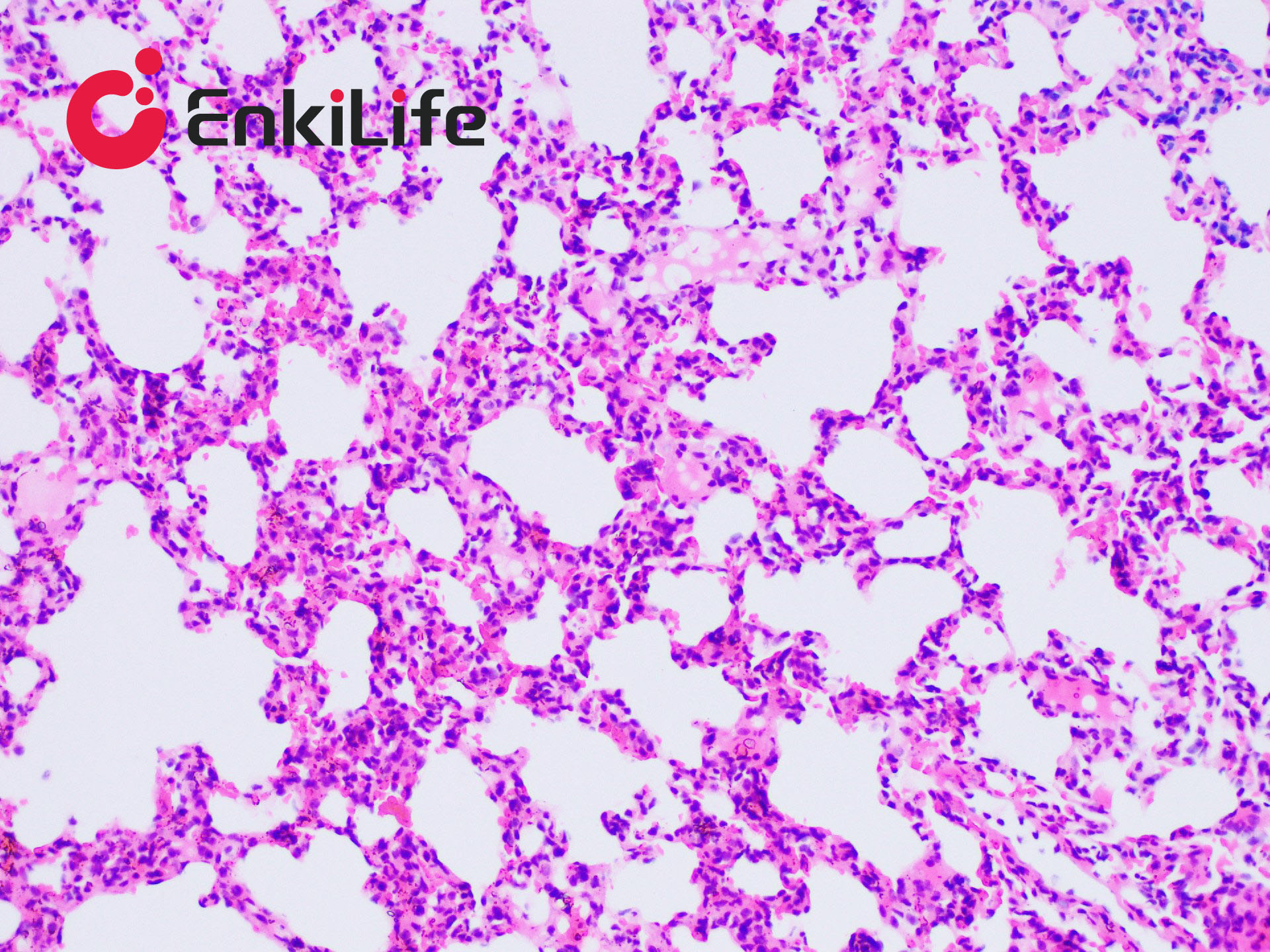
Calcium is abundant in the human body, forming the skeleton as a structural support. It also plays vital roles in secretion, transport, muscle contraction, and nerve conduction. Calcium exists in two forms: Ionic calcium – found in blood circulation (i.e., blood calcium); Bound calcium – combined with proteins, carbonates, or phosphates and deposited in tissues. Apart from bones and teeth, calcium is normally present in all tissues and cells, usually not in solid form. However, under certain pathological conditions, calcium may precipitate and deposit in tissues as solid particles, a process known as pathological calcification. The deposited calcium salts are mainly calcium phosphate, followed by calcium carbonate. Calcium salts are usually mono-refringent, except for calcium oxalate, which is bi-refringent. In H&E staining, calcium generally appears purple-blue. Many dyes can form chelates with calcium, including Alizarin Red S, Purpurin, and Nuclear Fast Red. Alizarin Red S, an anthraquinone derivative and sodium salt of alizarin sulfonate, can chelate with calcium in calcium carbonate or phosphate to form an orange-red complex.
Common calcium staining methods include the silver nitrate method and Alizarin Red S method. Von Kossa staining (silver method) is a metal substitution technique. When Von Kossa silver solution reacts with sections containing insoluble calcium salts, calcium is replaced by silver. Upon light exposure, the silver salt is reduced to black metallic silver, making this method suitable for high-throughput calcium staining in tissue sections.
Basic Information
Product name | Von Kossa Calcium Staining Kit |
Sizes | 2x 50 mL |
Storage | 2-8 ℃, keep away from light |
Shipping | Shipped with ice pack |
Validity | 12 months |
Product Components
Components | 2x 50mL |
Reagent (A): Von Kossa Silver Solution | 50 mL |
Reagent (B): Hypo Solution | 50 mL |
Reagent (C): Von Kossa Control Solution | 10 mL |
Notes
1. Neutral buffered formalin is the preferred fixative for calcium-containing tissues. Avoid acidic fixatives such as Bouin’s solution or calcium-formalin. If using routine 10% formalin, limit fixation to 4–6 h before dehydration to prevent acidification-induced calcium dissolution.
2. Reaction time depends on light intensity and exposure duration: 15 min under strong sunlight is usually sufficient; 10 min under UV light is adequate; Under normal indoor light, extend exposure time accordingly.
3. If calcium staining is too intense, dilute the Von Kossa Silver Solution with distilled water, or reduce light exposure time/intensity. If staining is too weak, increase the volume of silver solution and extend light exposure time and intensity.
4. This method can differentiate calcium salts from urates: Calcium salts are insoluble in lithium carbonate solution; Urates are soluble. Therefore, treat sections with lithium carbonate before Von Kossa staining and light exposure. Negative staining indicates urate deposition.
5. Use reagents promptly after opening to maintain optimal performance.